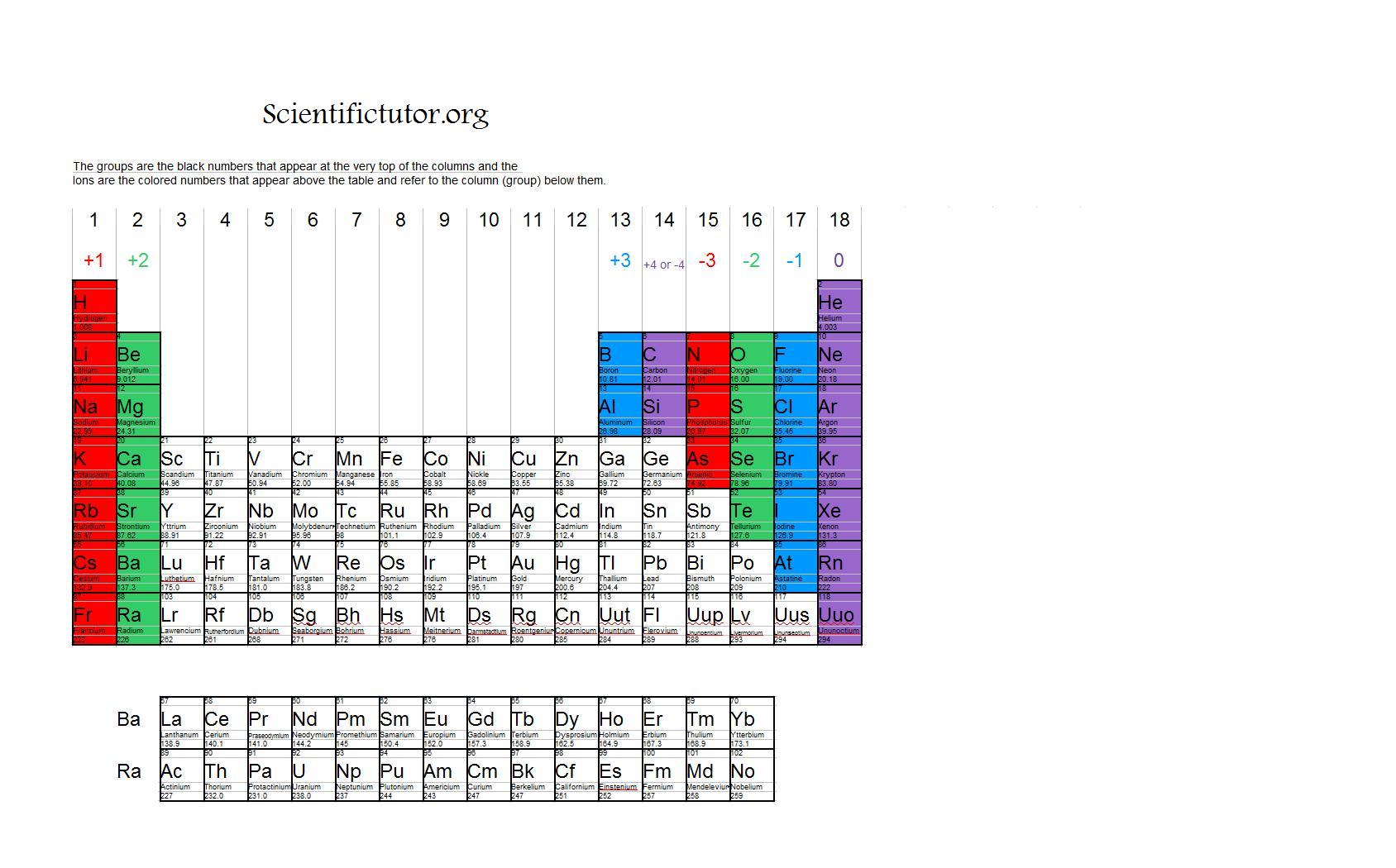
Which Group Tends To Form 1 Ions - Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of electron. They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react?
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Whats the opposite of electron. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react?
Whats the opposite of electron. Group 2 metals, the alkaline. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
Molecular and Ionic Compounds CHEM 1305 General Chemistry I—Lecture
Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline. They have 1 valance electron to lose to become a noble.
Question Video Connecting the Group Number with the Subscript in an
Group 2 metals, the alkaline. They have 1 valance electron to lose to become a noble gas. Which group tends to not form ions or react? Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized.
Is Oxygen a Positive or Negative Ion? Infrared for Health
Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group tends to not form ions or react? They have 1 valance electron to lose to become a noble gas. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+.
Ionic Compound Nomenclature Presentation Chemistry
Whats the opposite of electron. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas.
O Ion 19 39k+1 Possui
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the alkaline. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Which group on the periodic table tends to form 1+ ions? Which group.
Solved Sort the following statements based on whether demand
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. They have 1 valance electron to lose to become a noble gas. Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? Study with quizlet and memorize flashcards containing terms like which group tends to form.
What Is The Charge Of Group 13 On Periodic Table
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends.
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. They have 1 valance electron to lose to become a noble gas. Study with quizlet and memorize flashcards containing terms like which group tends to form 1+ ions?, which group tends to form 2+ ions?, which. Group 2 metals, the alkaline. Whats.
Metals and nonmetals The Basics Reactivity Reactions with
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Whats the opposite of electron. Group 2 metals, the alkaline. Which group on the periodic table tends to form 1+ ions? They have 1 valance electron to lose to become a noble gas.
SOLVED ATOMIC RADIUS What trend in atomic radius do you see as you go
They have 1 valance electron to lose to become a noble gas. Which group on the periodic table tends to form 1+ ions? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m^+ ions when oxidized. Group 2 metals, the alkaline. Which group tends to not form ions or react?
Study With Quizlet And Memorize Flashcards Containing Terms Like Which Group Tends To Form 1+ Ions?, Which Group Tends To Form 2+ Ions?, Which.
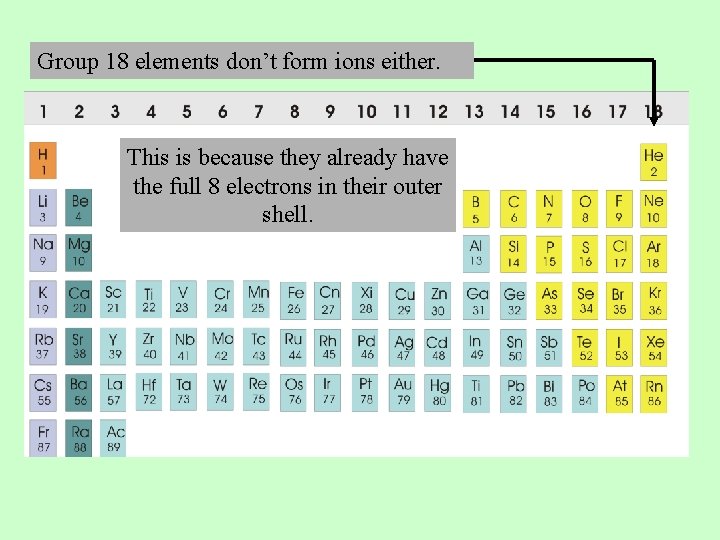
Which group tends to not form ions or react? They have 1 valance electron to lose to become a noble gas. Which group on the periodic table tends to form 1+ ions? Group 2 metals, the alkaline.
Group 1 Metals, The Alkali Metals, Have The 1 Valence Electron, And Thus Form M^+ Ions When Oxidized.
Whats the opposite of electron.



.PNG)