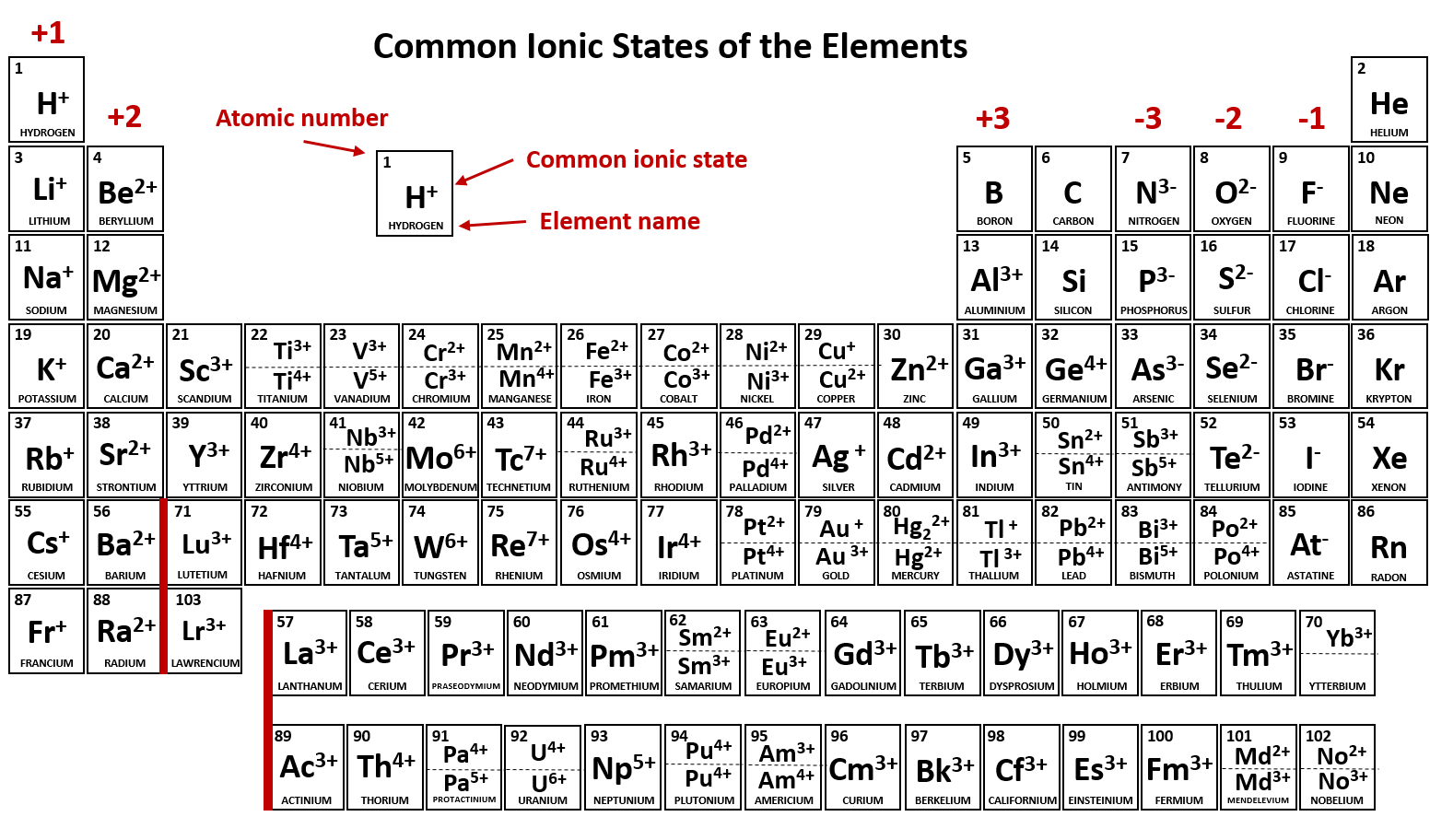
Which Element Is Most Likely To Form A 2 Ion - Their atoms have 6 valence electrons, and need 2 more to. (review the periodic table to view the elements) iodine and potassium form an ionic. The elements that are most likely to form 2− ions are the group 16 elements. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Which two elements will most likely form an ionic bond?
(review the periodic table to view the elements) iodine and potassium form an ionic. Their atoms have 6 valence electrons, and need 2 more to. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Which two elements will most likely form an ionic bond? The elements that are most likely to form 2− ions are the group 16 elements.
Which two elements will most likely form an ionic bond? The elements that are most likely to form 2− ions are the group 16 elements. (review the periodic table to view the elements) iodine and potassium form an ionic. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Their atoms have 6 valence electrons, and need 2 more to.
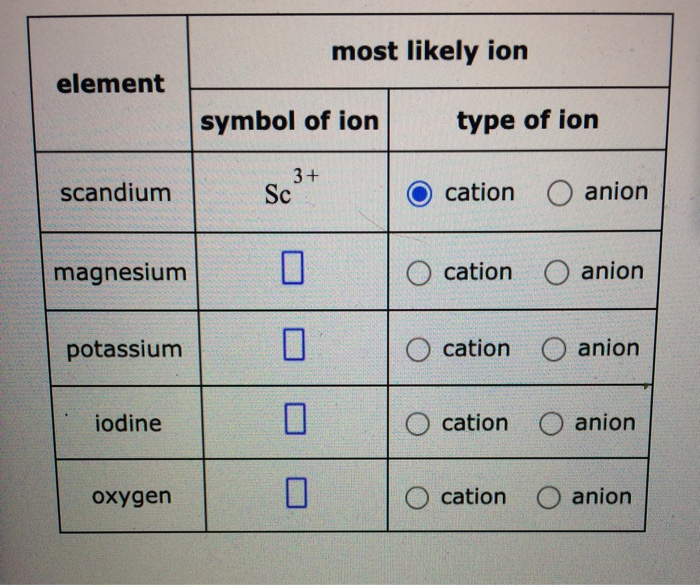
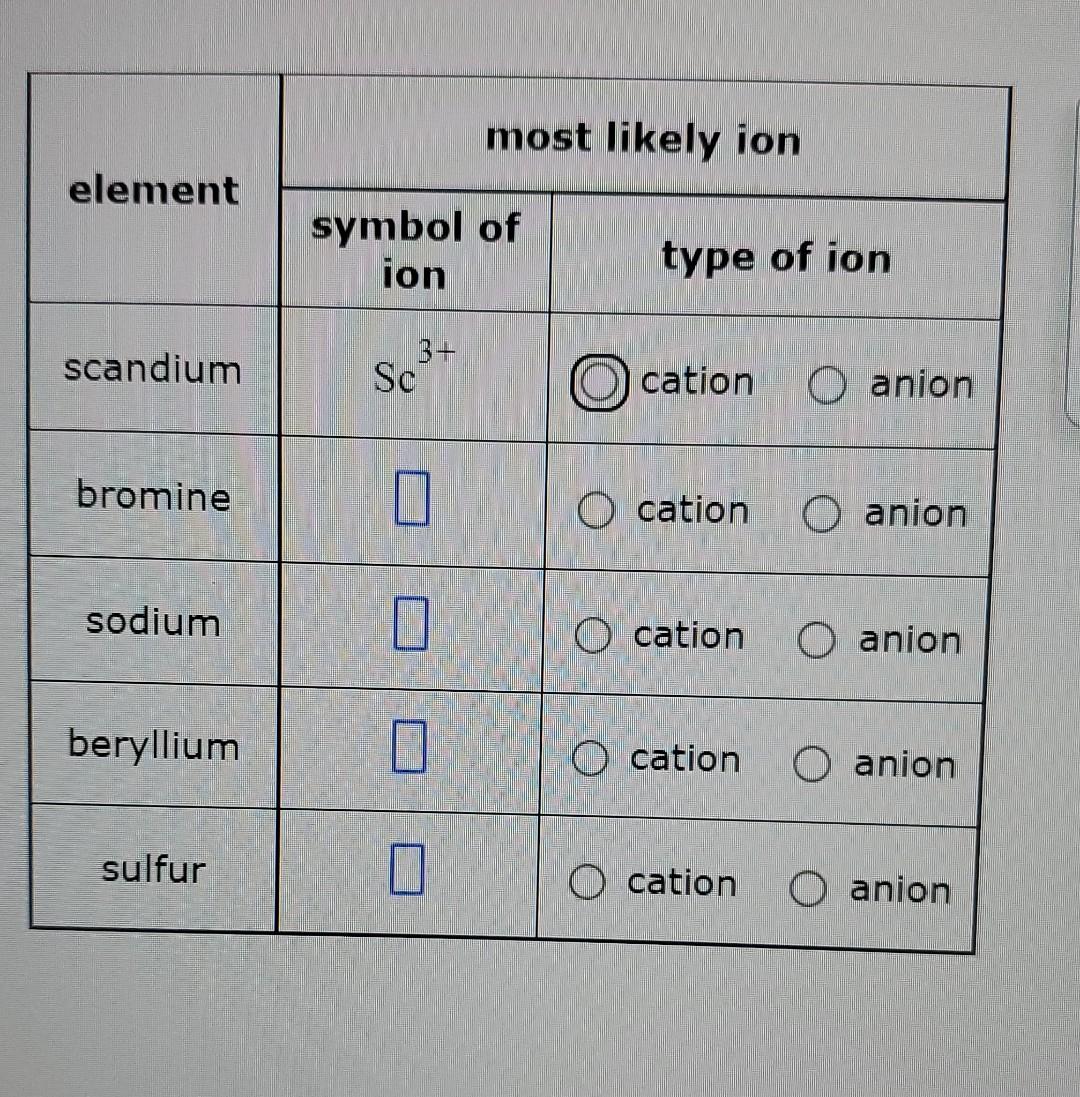
SOLVED most likely ion element symbol of ion type of ion scandium Sc3
Their atoms have 6 valence electrons, and need 2 more to. The elements that are most likely to form 2− ions are the group 16 elements. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Which two elements will most likely form an ionic bond?.
Solved 11. Which of the following elements is most likely to
Which two elements will most likely form an ionic bond? The elements that are most likely to form 2− ions are the group 16 elements. Their atoms have 6 valence electrons, and need 2 more to. (review the periodic table to view the elements) iodine and potassium form an ionic. Study with quizlet and memorize flashcards containing terms like which.
metals tend to form what kind of ions Lombardi Bothe1936
The elements that are most likely to form 2− ions are the group 16 elements. Which two elements will most likely form an ionic bond? Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Their atoms have 6 valence electrons, and need 2 more to..
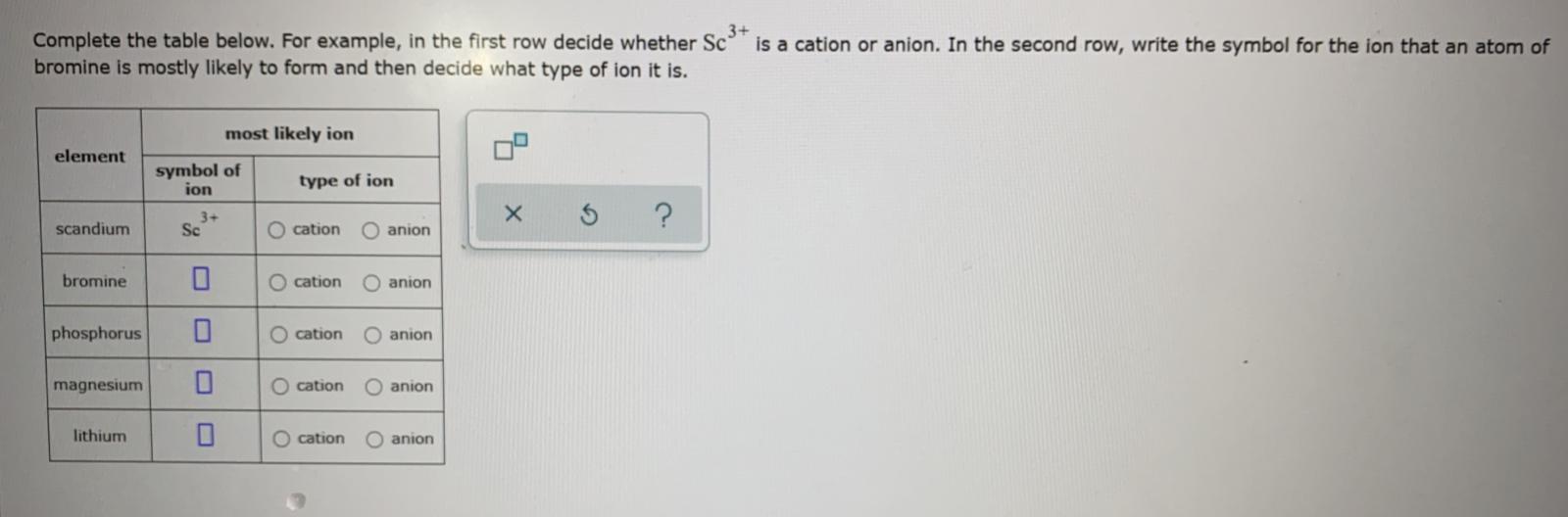
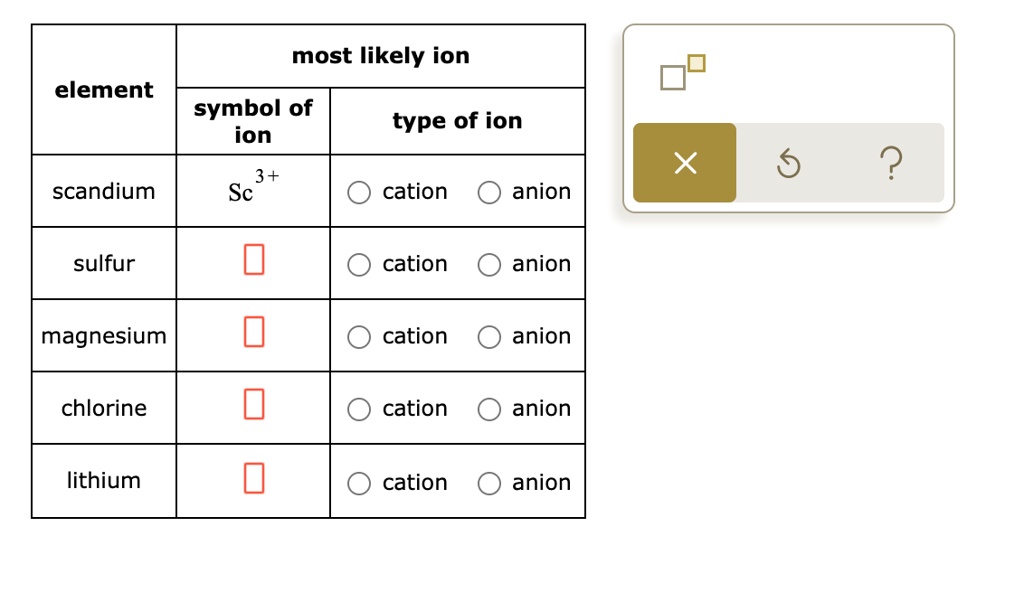
Solved Complete the table below. For example, in the first
Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Which two elements will most likely form an ionic bond? Their atoms have 6 valence electrons, and need 2 more to. The elements that are most likely to form 2− ions are the group 16 elements..
Which of the Following Elements Form +2 Ions
Which two elements will most likely form an ionic bond? Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. (review the periodic table to view the elements) iodine and potassium form an ionic. Their atoms have 6 valence electrons, and need 2 more to. The.
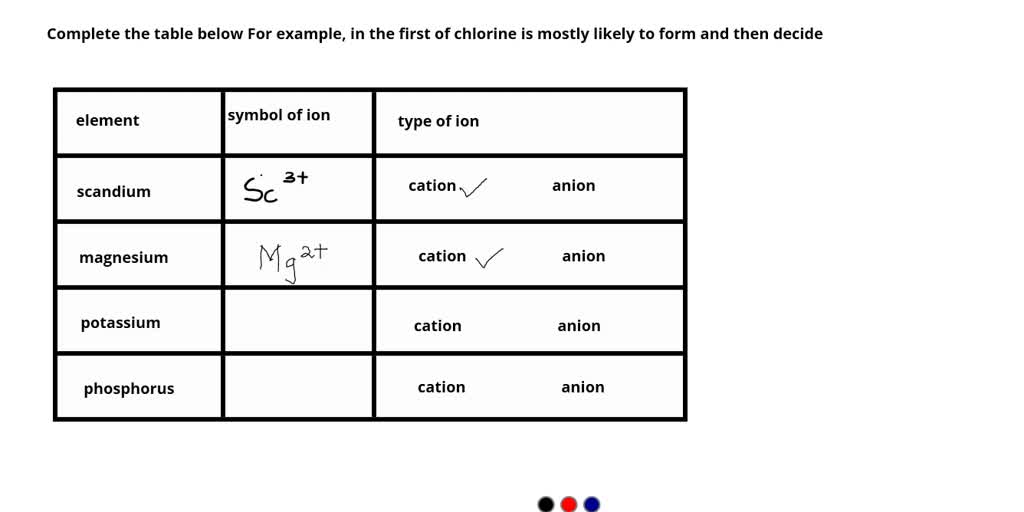
SOLVED Complete the table below For example, in the first of chlorine
(review the periodic table to view the elements) iodine and potassium form an ionic. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. Which two elements will most likely form an ionic bond? Their atoms have 6 valence electrons, and need 2 more to. The.
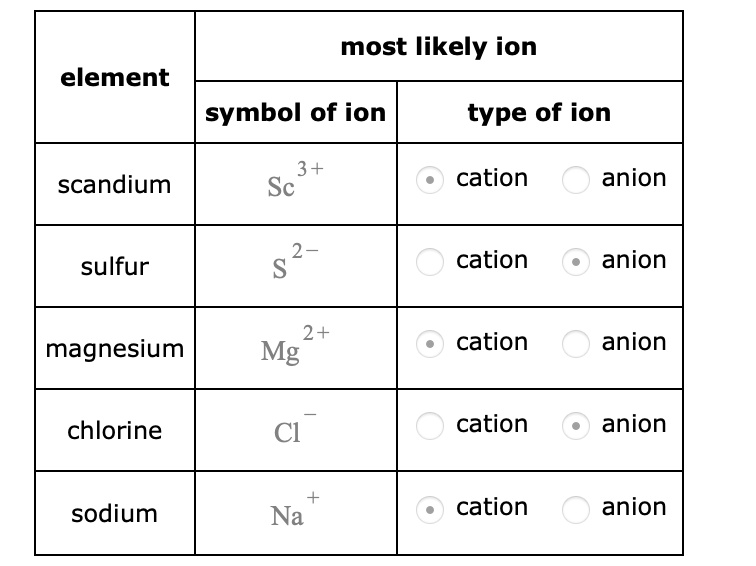
Solved most likely ion element symbol of ion type of ion
Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. The elements that are most likely to form 2− ions are the group 16 elements. Which two elements will most likely form an ionic bond? (review the periodic table to view the elements) iodine and potassium.
What Are the 3 Major Ions? Infrared for Health
Which two elements will most likely form an ionic bond? The elements that are most likely to form 2− ions are the group 16 elements. Their atoms have 6 valence electrons, and need 2 more to. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2..
Solved most likely ion element symbol of ion type of ion
The elements that are most likely to form 2− ions are the group 16 elements. (review the periodic table to view the elements) iodine and potassium form an ionic. Which two elements will most likely form an ionic bond? Their atoms have 6 valence electrons, and need 2 more to. Study with quizlet and memorize flashcards containing terms like which.
SOLVED most likely ion element symbol of ion type of ion 3 + Sc
The elements that are most likely to form 2− ions are the group 16 elements. Which two elements will most likely form an ionic bond? (review the periodic table to view the elements) iodine and potassium form an ionic. Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion.
Their Atoms Have 6 Valence Electrons, And Need 2 More To.
Study with quizlet and memorize flashcards containing terms like which of the following elements is most likely to form an ion with a +2. The elements that are most likely to form 2− ions are the group 16 elements. (review the periodic table to view the elements) iodine and potassium form an ionic. Which two elements will most likely form an ionic bond?