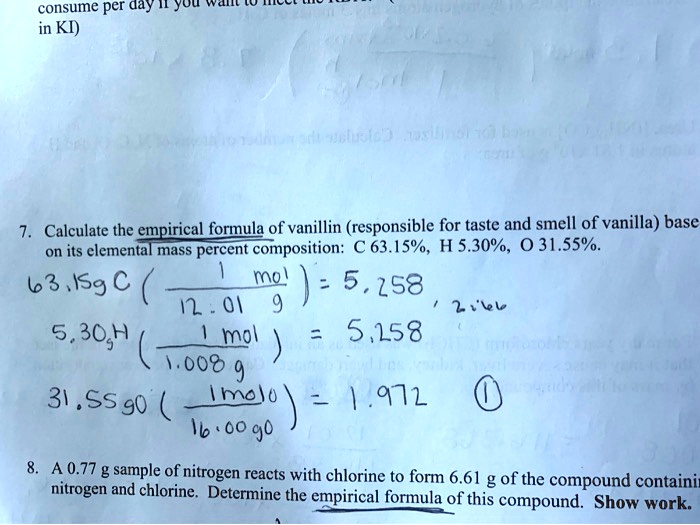What Is The Empirical Formula Of Vanillin - What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Calculation of the number of moles of c, h, and o in vanillin: The empirical formula of vanillin: Vanillin, the dominant flavoring in vanilla, contains c, h, and o. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What is the empirical formula?
The empirical formula of vanillin: A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What is the empirical formula? Vanillin, the dominant flavoring in vanilla, contains c, h, and o. Calculation of the number of moles of c, h, and o in vanillin:
A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, the dominant flavoring in vanilla, contains c, h, and o. The empirical formula of vanillin: Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What is the empirical formula? Calculation of the number of moles of c, h, and o in vanillin: When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#?
Solved Determine the empirical formula of vanillin, a
Vanillin, a flavoring agent, is made up of carbon, hydrogen,. The empirical formula of vanillin: Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent.
[Solved] Empirical Formula Vanillin is the compound containing C, H
When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What is the empirical formula? Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#?
15 Astounding Facts About Empirical Formula
Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin: Calculation of the number of moles of c, h, and o in vanillin:
[Solved] Empirical Formula Vanillin is the compound containing C, H
What is the empirical formula? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? The empirical formula of vanillin:
SOLVED Calculate the empirical formula for each natural flavor based
The empirical formula of vanillin: Calculation of the number of moles of c, h, and o in vanillin: When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. Vanillin, a flavoring agent, is made up of carbon, hydrogen,.
What is the empirical formula of Vanillin? HIX Tutor
The empirical formula of vanillin: What is the empirical formula? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g.
SOLVED 7.0 Calculate the empirical formula of vanillin (responsible
Calculation of the number of moles of c, h, and o in vanillin: A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Vanillin, the dominant flavoring in vanilla, contains c, h, and.
SOLVEDThe flavoring agent vanillin contains carbon, hydrogen, and
Vanillin, a flavoring agent, is made up of carbon, hydrogen,. Vanillin, the dominant flavoring in vanilla, contains c, h, and o. A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? The empirical formula of vanillin:
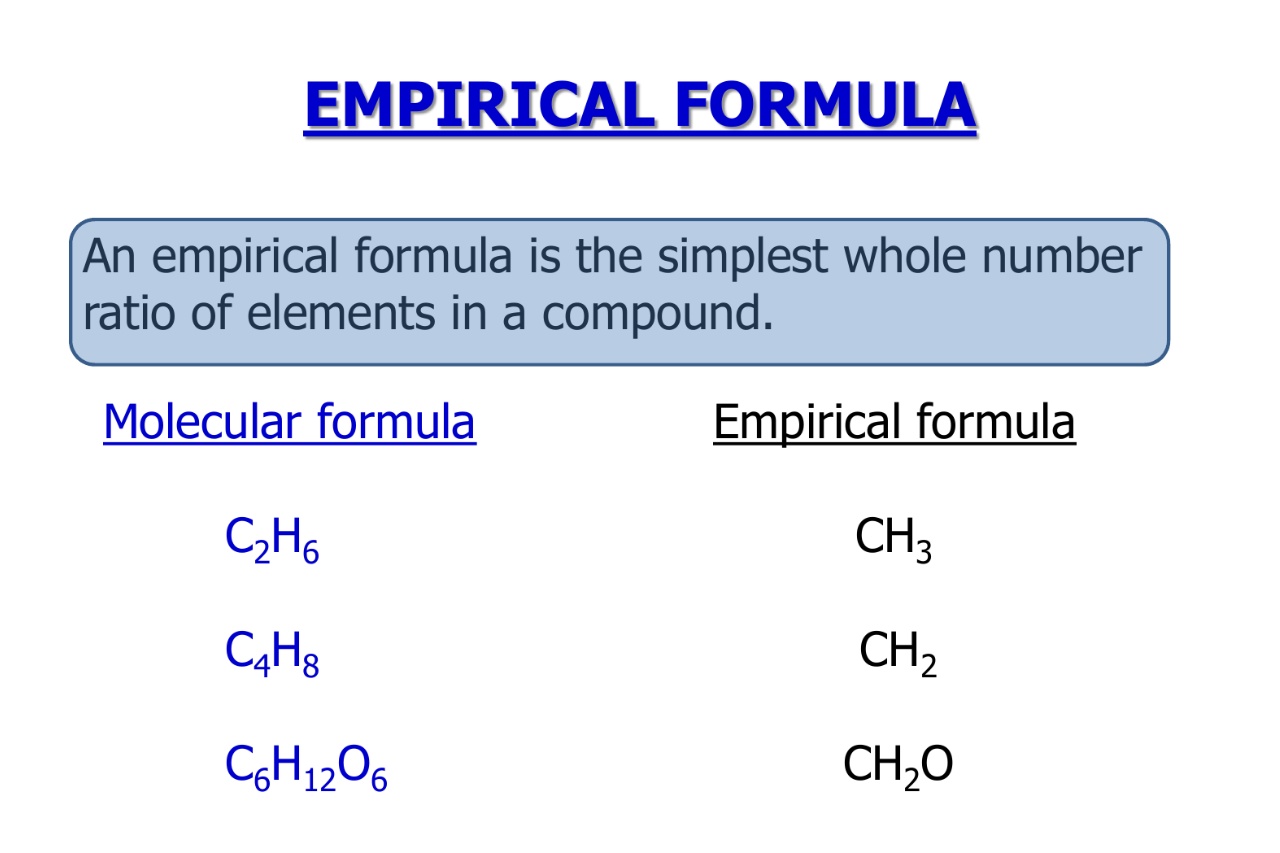
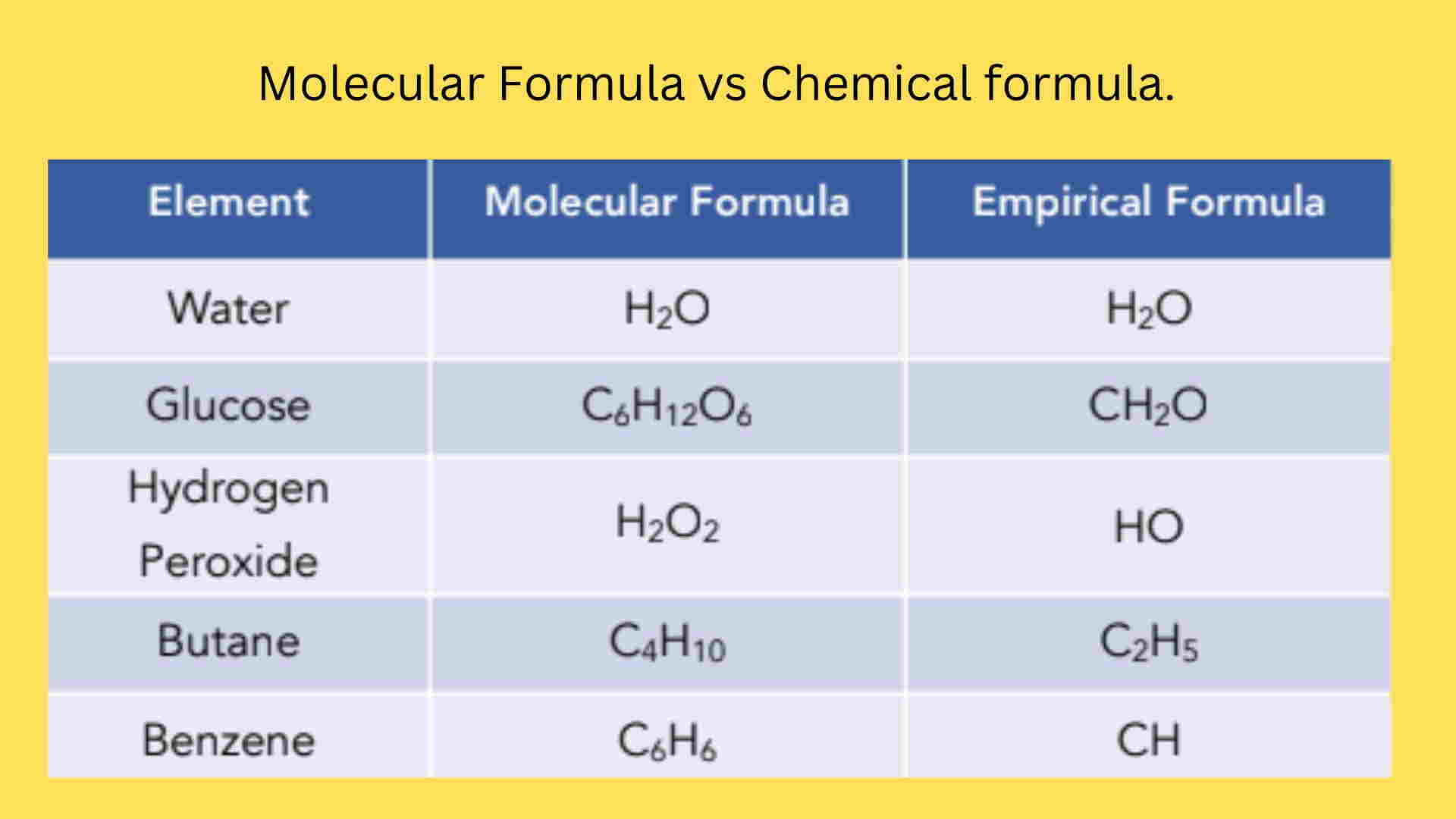
Empirical Formula, Molecular Formula Examples, Calculations
What is the empirical formula? What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? The empirical formula of vanillin: A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g.
[Solved] Empirical Formula Vanillin is the compound containing C, H
Vanillin, the dominant flavoring in vanilla, contains c, h, and o. What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. The empirical formula of vanillin: Calculation of the number of moles of c, h, and o in vanillin:
Calculation Of The Number Of Moles Of C, H, And O In Vanillin:
What is the empirical formula? What are the empirical formula and empirical formula mass for #c_8h_24 0_8#? Vanillin, the dominant flavoring in vanilla, contains c, h, and o. Vanillin, a flavoring agent, is made up of carbon, hydrogen,.
The Empirical Formula Of Vanillin:
A laboratory analysis of vanillin, the flavoring agent in vanilla, determined the following mass percent. When 1.05 g of this substance is completely combusted, 2.43 g of co2 and 0.50 g.