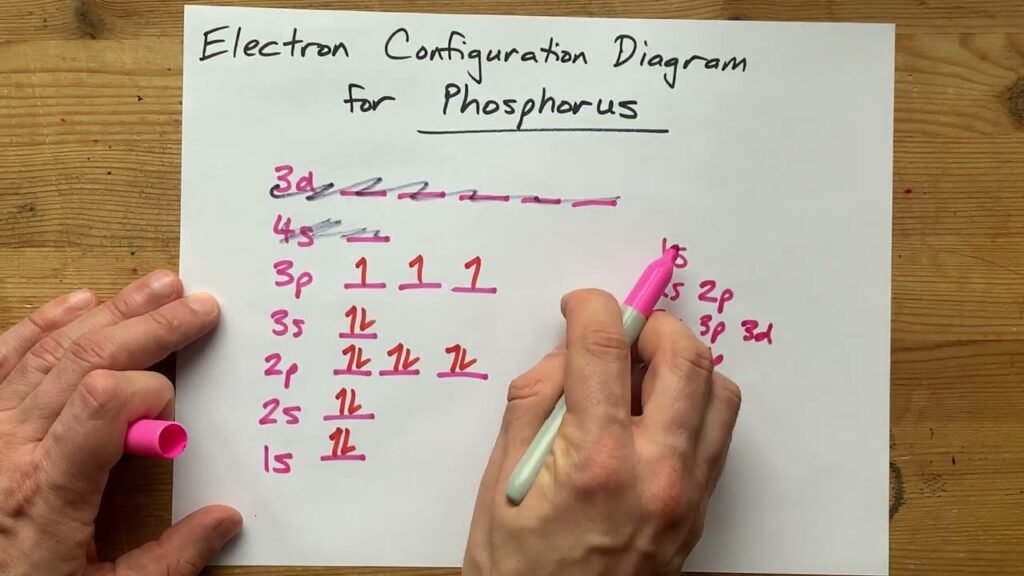
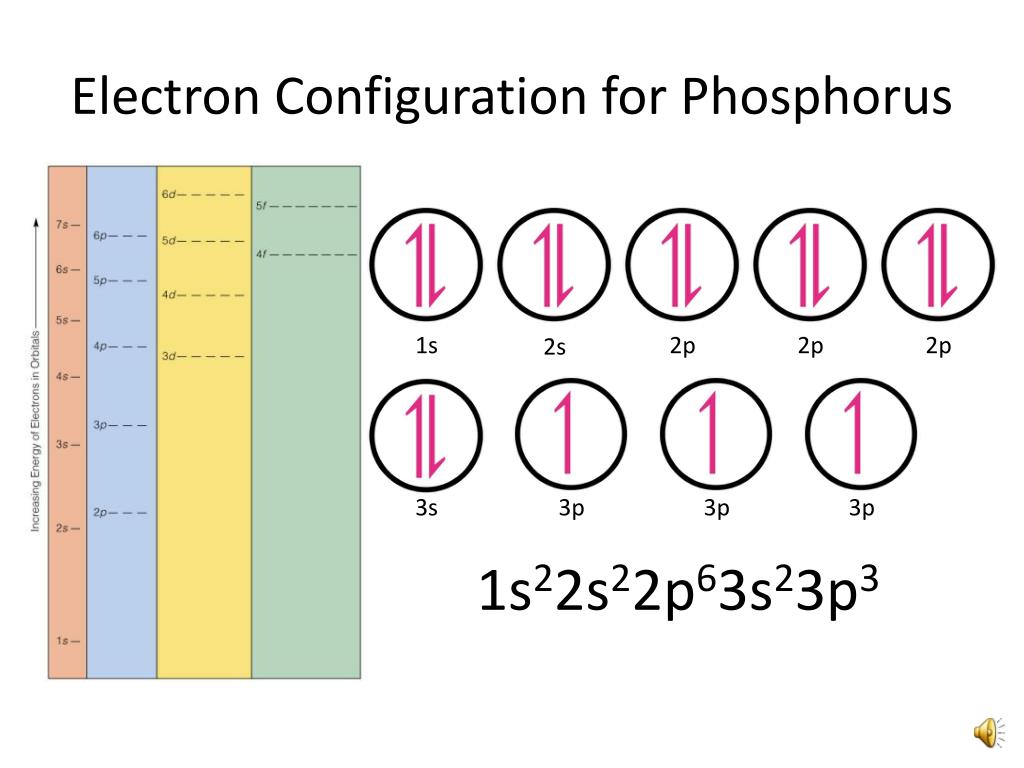
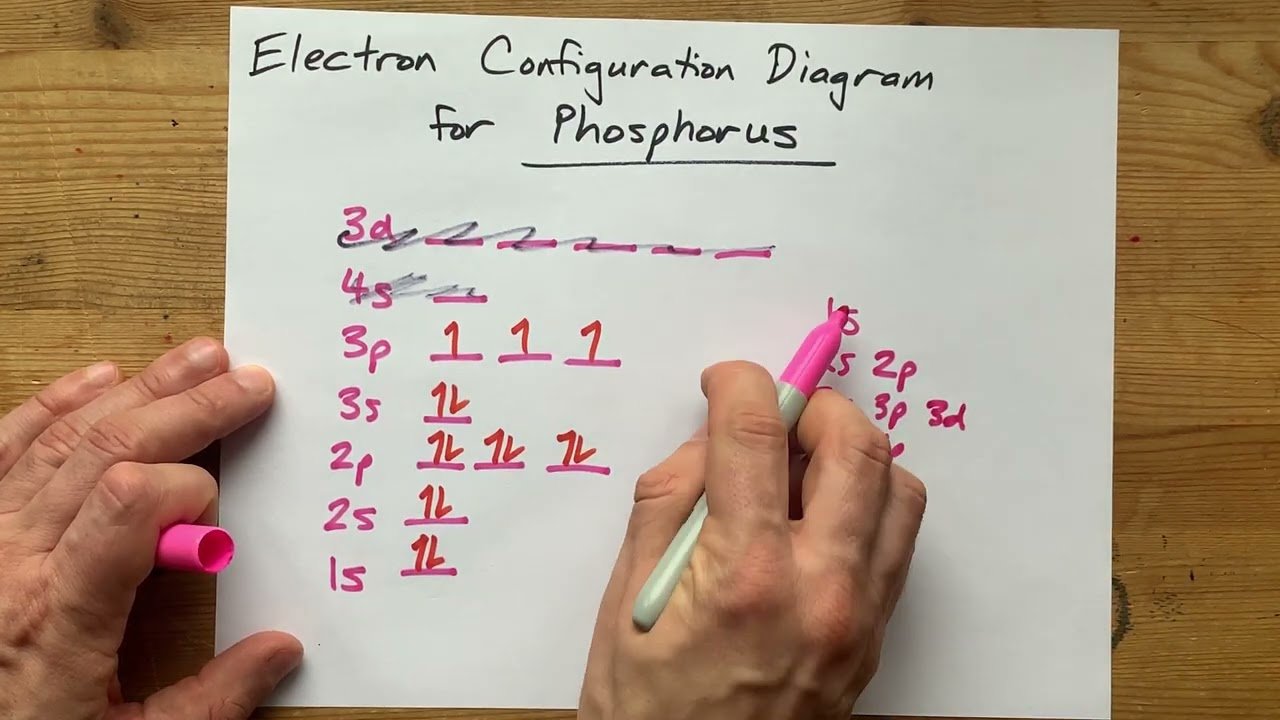
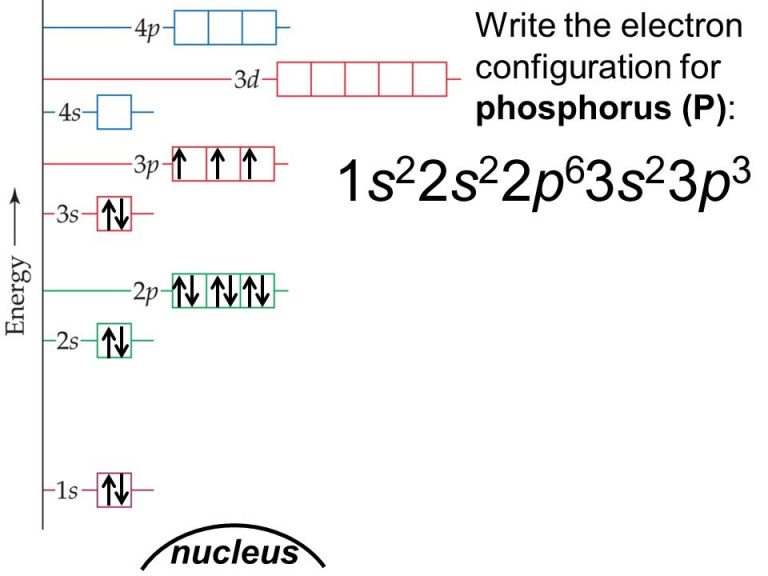
What Is The Correct Electron Configuration Of Phosphorus P - 2 electrons in the 3. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. The valence electron configuration for phosphorus is s^2 p^3. This means that phosphorus has 15 electrons arranged in different. The shorthand electron configuration (or noble gas. The electron configuration of an element represents the.
Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. The electron configuration of an element represents the. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). This means that phosphorus has 15 electrons arranged in different. 2 electrons in the 3. The shorthand electron configuration (or noble gas. The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. Electron configuration chart of all elements is mentioned in the table below. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. The valence electron configuration for phosphorus is s^2 p^3.
The valence electron configuration for phosphorus is s^2 p^3. The shorthand electron configuration (or noble gas. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. 2 electrons in the 3. The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. This means that phosphorus has 15 electrons arranged in different. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). The electron configuration of an element represents the. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3.
Lewis Dot Symbol For The Phosphorus Ion
The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. This means that phosphorus has 15 electrons arranged in different. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. The valence electron configuration for phosphorus is s^2 p^3. Electron configuration chart of all elements is mentioned in the table below.
The Electron Configuration of Phosphorus Unveiling its Atomic
2 electrons in the 3. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. The electron configuration of an element represents the. The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. The valence electron configuration for phosphorus is s^2 p^3.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
Electron configuration chart of all elements is mentioned in the table below. The electron configuration of an element represents the. This means that phosphorus has 15 electrons arranged in different. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³.
【5 Steps】Electron Configuration for Phosphorus (P) in Just 5 Steps
The shorthand electron configuration (or noble gas. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3.
Phosphorus P (Element 15) of Periodic Table Elements FlashCards
The electron configuration of an element represents the. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. This means that phosphorus has 15 electrons arranged in different. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). The correct electron.
The Electron Configuration of Phosphorus Unveiling its Atomic
In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). The electron configuration of an element represents the. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. This means that phosphorus has 15 electrons arranged in different. The valence electron configuration for phosphorus.
Orbital Diagram For Phosphorus
In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). Electron configuration chart of all elements is mentioned in the table below. This means that phosphorus has 15 electrons arranged in different. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. The electron configuration.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. Electron configuration chart of all elements is mentioned in the table below. 2 electrons in the 3. The valence electron configuration for phosphorus is s^2 p^3.
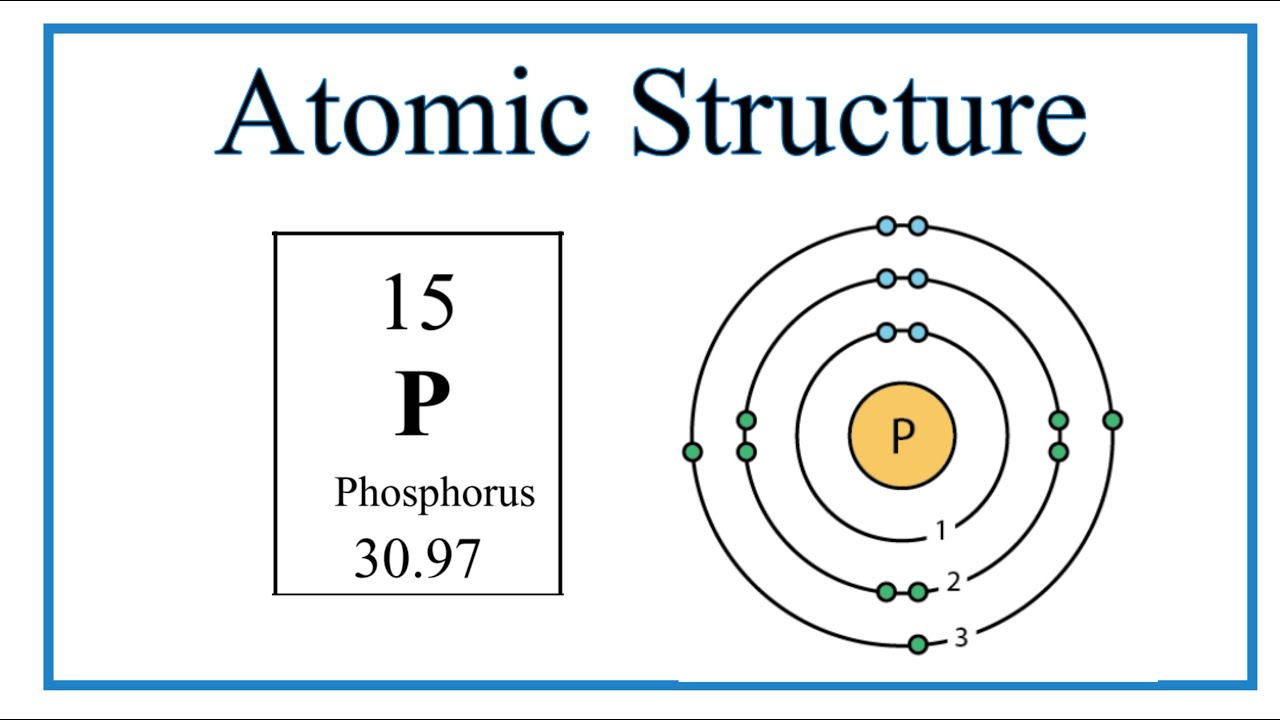
How To Make A Phosphorus Atom Model Design Talk
The shorthand electron configuration (or noble gas. This means that phosphorus has 15 electrons arranged in different. In order to write the phosphorus electron configuration we first need to know the number of electrons for the p atom (there are 15 electrons). The correct electron configuration of phosphorus (p) is (b) 1s²2s²2p⁶3s²3p³. The valence electron configuration for phosphorus is s^2.
Phosphorus Electron Configuration (P) with Orbital Diagram
The correct electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3. Electron configuration chart of all elements is mentioned in the table below. This means that phosphorus has 15 electrons arranged in different. The shorthand electron configuration (or noble gas. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3.
In Order To Write The Phosphorus Electron Configuration We First Need To Know The Number Of Electrons For The P Atom (There Are 15 Electrons).
The valence electron configuration for phosphorus is s^2 p^3. Phosphorus has an electron configuration of 1s^2 2s^2 2p^6, 3s^2 3p^3. Phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. This means that phosphorus has 15 electrons arranged in different.
The Correct Electron Configuration Of Phosphorus Is 1S2 2S2 2P6 3S2 3P3.
The shorthand electron configuration (or noble gas. 2 electrons in the 3. The electron configuration of an element represents the. Electron configuration chart of all elements is mentioned in the table below.