Usfda Form 483 - During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
USFDA issues Form483 with 3 observations to Alkem Lab's St Louis plant
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
Dr Reddy's Labs shares in red after USFDA issues form 483 with four
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
483 Inspection Observation Responses Customs & International Trade
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
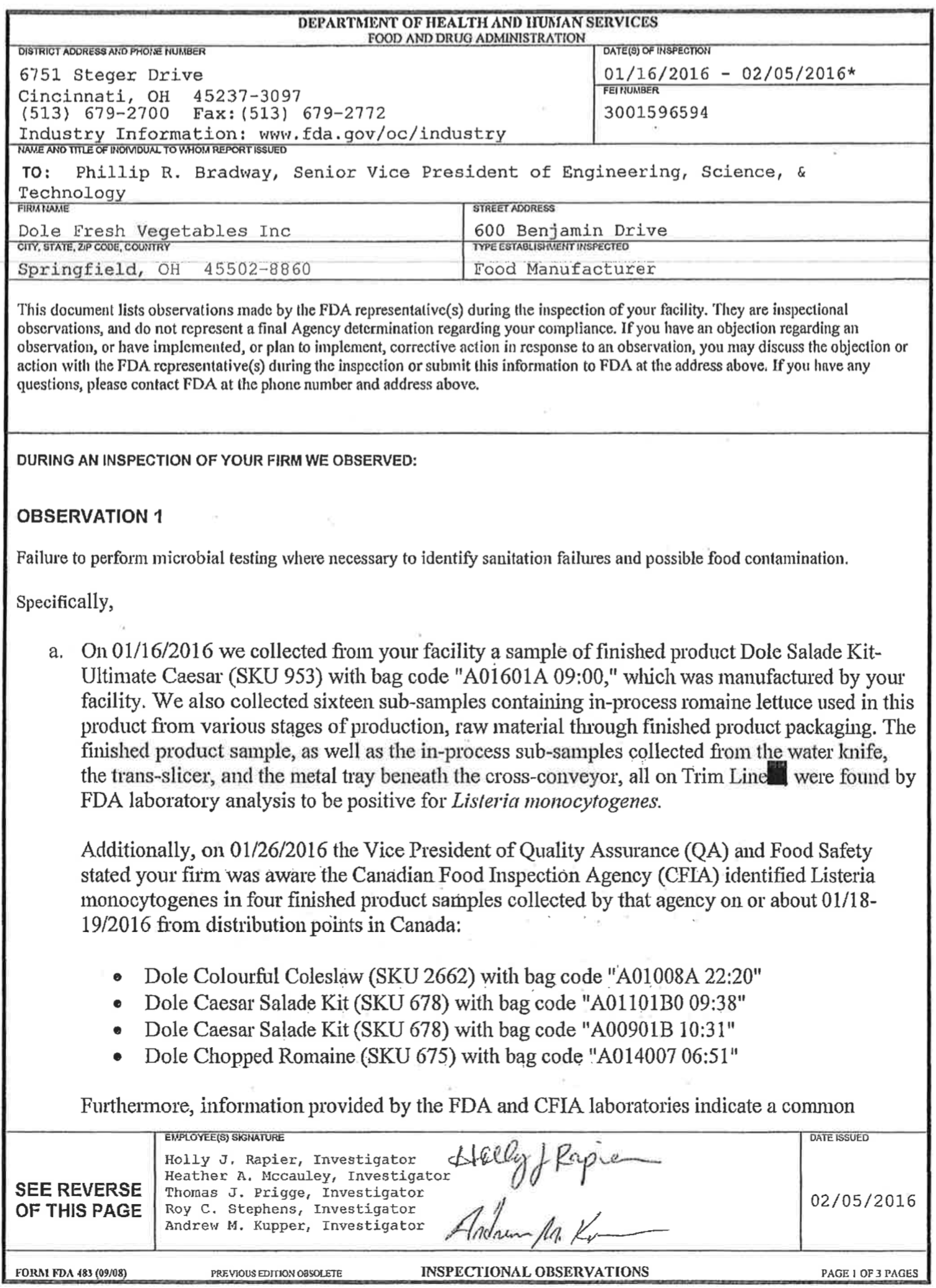
Dole’s FDA 483 Window into Lettuce Production Marler Blog
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
USFDA issues Form483 with 1 observation to Torrent Pharma's Gujarat
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
USFDA issues Form 483 with five inspectional observations to Marksans
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
USFDA Inspection Form 482, Form 483 & Form 484
During an inspection, ora investigators may observe conditions they deem to be objectionable. A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.
What is USFDA’s Form 483? YouTube
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
USFDA issues Form483 with 8 observations to Lupin's drug, API
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in. During an inspection, ora investigators may observe conditions they deem to be objectionable.
During An Inspection, Ora Investigators May Observe Conditions They Deem To Be Objectionable.
A form fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed conditions that in.