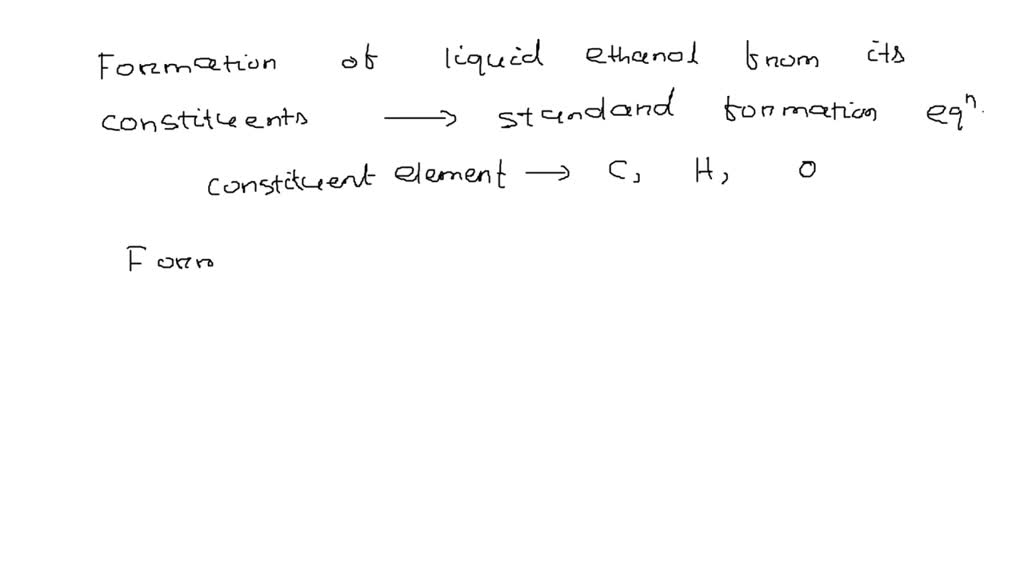
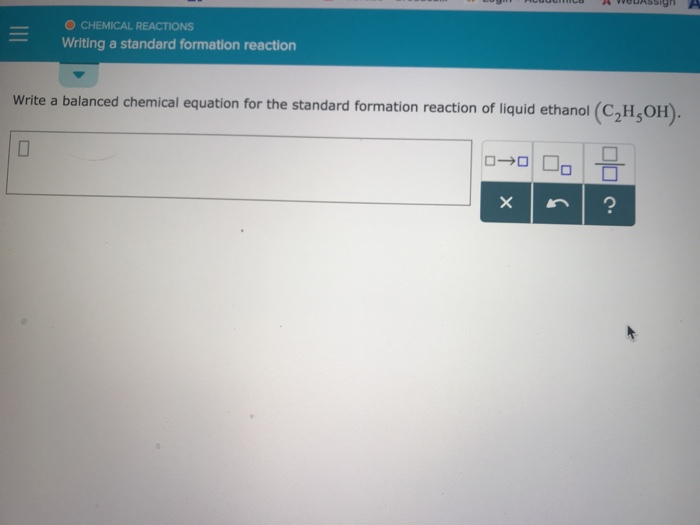
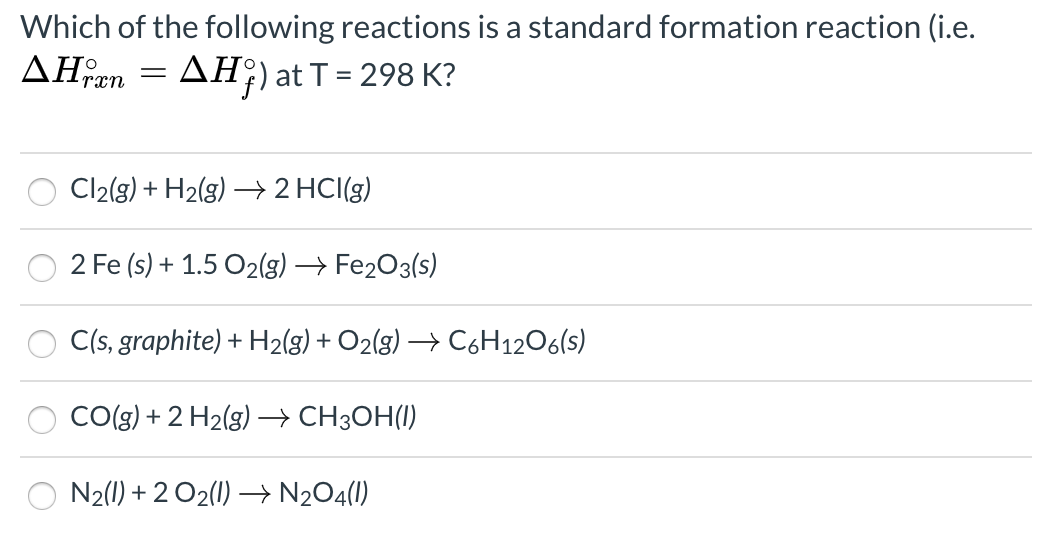Standard Formation Reaction Of Liquid Ethanol - What is the standard enthalpy of formation for ethanol c 2h 5oh? The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Determine the standard enthalpy of formation for ethylene glycol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 1) the first thing to do is look up standard enthalpies of formation for the. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.
Determine the standard enthalpy of formation for ethylene glycol. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 1) the first thing to do is look up standard enthalpies of formation for the. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. What is the standard enthalpy of formation for ethanol c 2h 5oh? Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1.
Determine the standard enthalpy of formation for ethylene glycol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 1) the first thing to do is look up standard enthalpies of formation for the. What is the standard enthalpy of formation for ethanol c 2h 5oh?
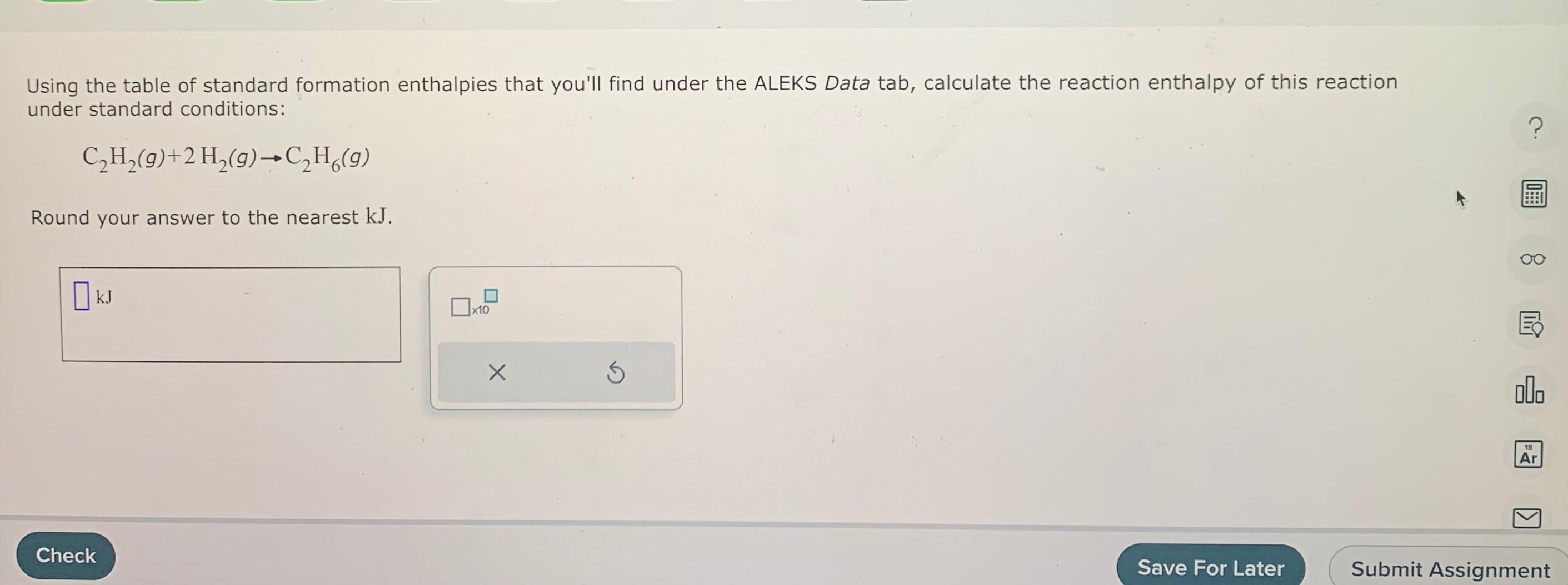
Solved Using the table of standard formation enthalpies that
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. What is the standard enthalpy of formation for ethanol c 2h 5oh? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation.
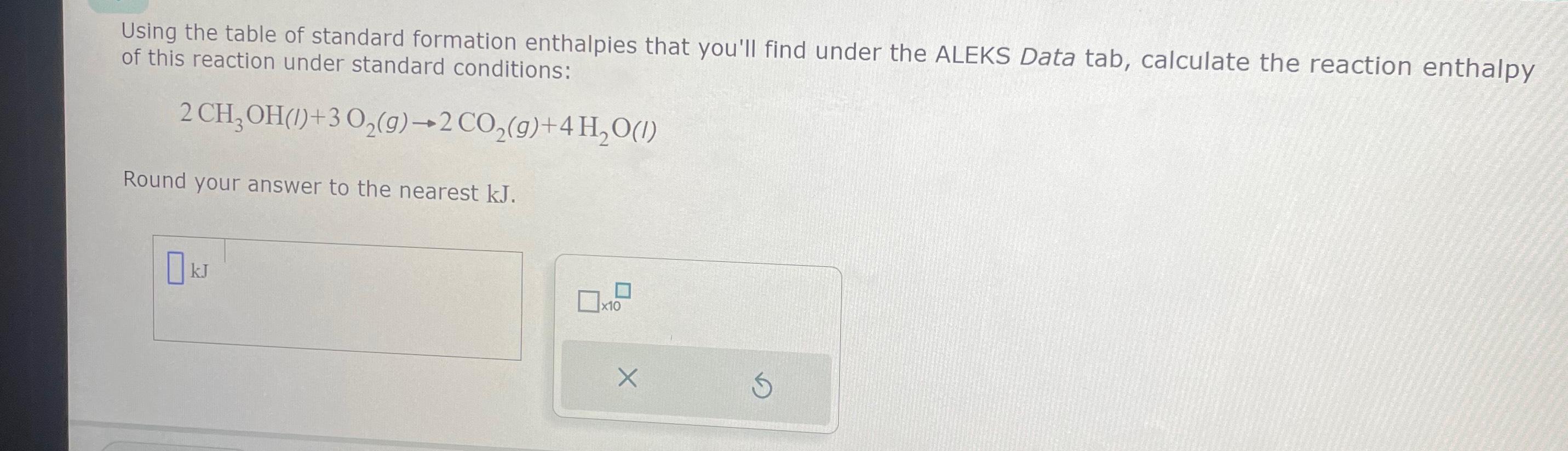
Solved Using the table of standard formation enthalpies that
What is the standard enthalpy of formation for ethanol c 2h 5oh? Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy.
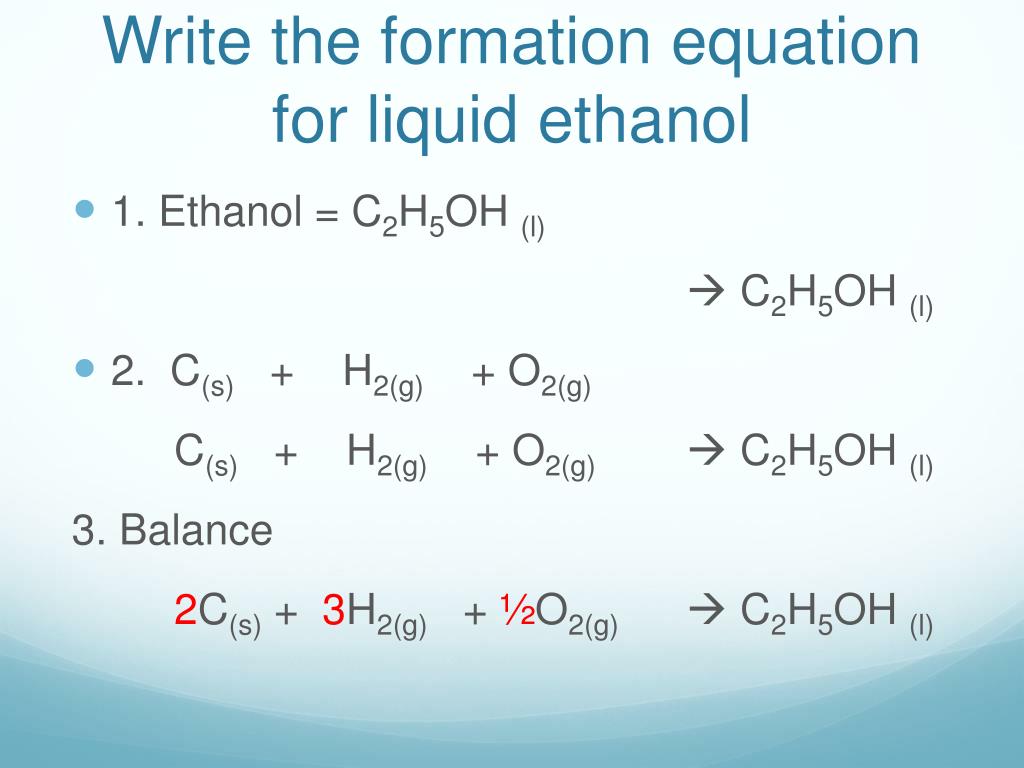
PPT Heat of Formation PowerPoint Presentation, free download ID3890043
1) the first thing to do is look up standard enthalpies of formation for the. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Determine the standard enthalpy of formation for ethylene glycol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or.
Solved Which of the following reactions is a standard
1) the first thing to do is look up standard enthalpies of formation for the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Determine the.
SOLVED6. Calculate the standard enthalpy change (AH) for the
The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. Determine the standard enthalpy of formation for ethylene glycol. 1) the first thing to do is look up standard enthalpies of.
Vidéo question Déterminer l’enthalpie standard de formation de l
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Determine the standard enthalpy of formation for ethylene glycol. What is the standard enthalpy of formation for.
SOLVED 'Write a balanced chemical equation for the standard formation
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Determine the standard enthalpy of formation for ethylene glycol. Well, this site quotes δh ∘.
SOLVED a) Write a balanced chemical equation for the standard enthalpy
What is the standard enthalpy of formation for ethanol c 2h 5oh? 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. 1) the first thing to do is look up standard enthalpies.
Solved O CHEMICAL REACTIONS Writing a standard formation
1) the first thing to do is look up standard enthalpies of formation for the. What is the standard enthalpy of formation for ethanol c 2h 5oh? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Balanced chemical equation for the formation of ethanol the standard.
Standard Enthalpy of Formation and Formation Reactions OpenStax
What is the standard enthalpy of formation for ethanol c 2h 5oh? The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states. Determine the standard enthalpy of formation for ethylene glycol. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1..
Determine The Standard Enthalpy Of Formation For Ethylene Glycol.
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Balanced chemical equation for the formation of ethanol the standard formation reaction of liquid ethanol (c2h5oh) involves the combination of. Well, this site quotes δh ∘ f (ethanol) = − 277.7 ⋅ kj ⋅ mol−1. The standard enthalpy of formation, δh o f, is the enthalpy change for a formation equation when all substances are in their standard states.
1) The First Thing To Do Is Look Up Standard Enthalpies Of Formation For The.
136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. What is the standard enthalpy of formation for ethanol c 2h 5oh?