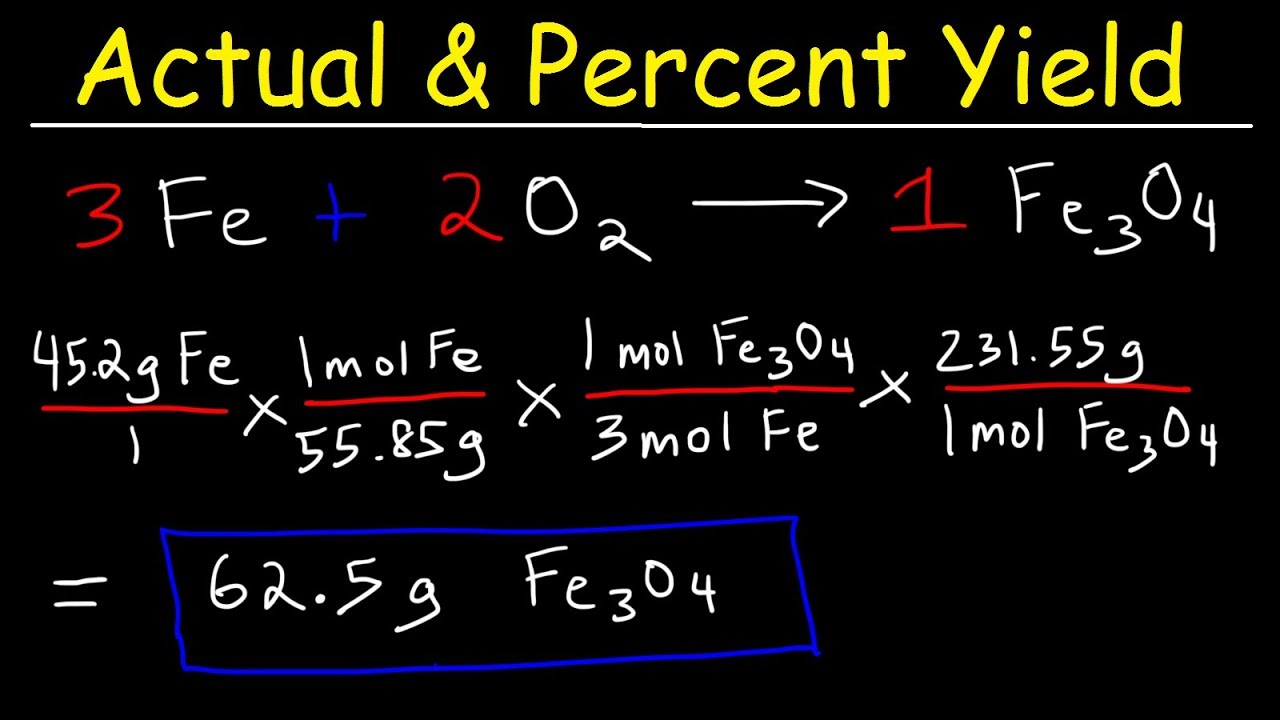
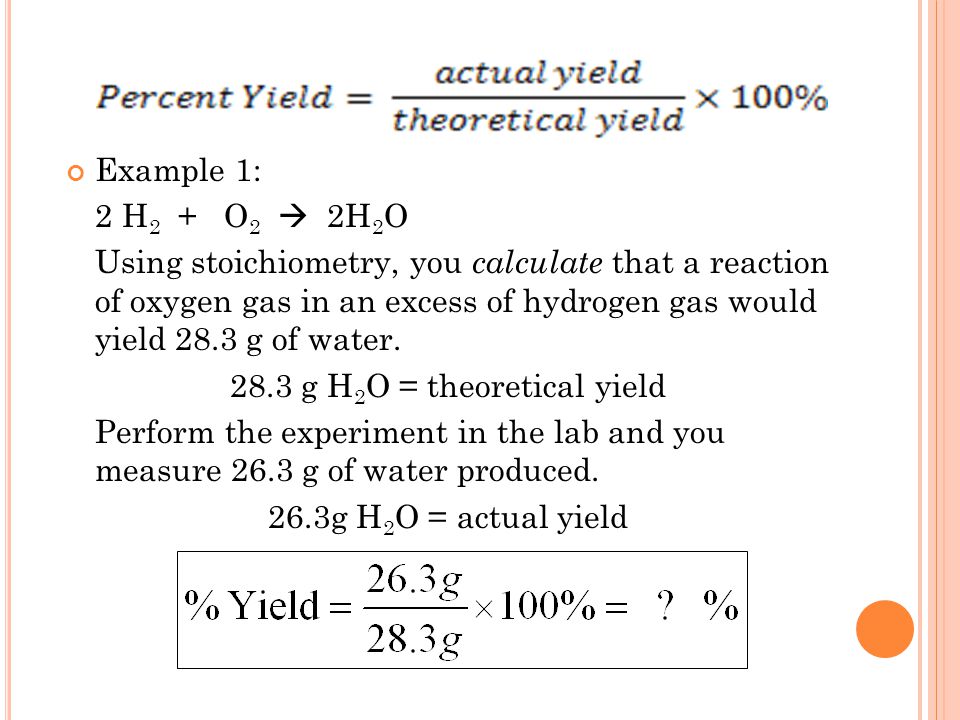
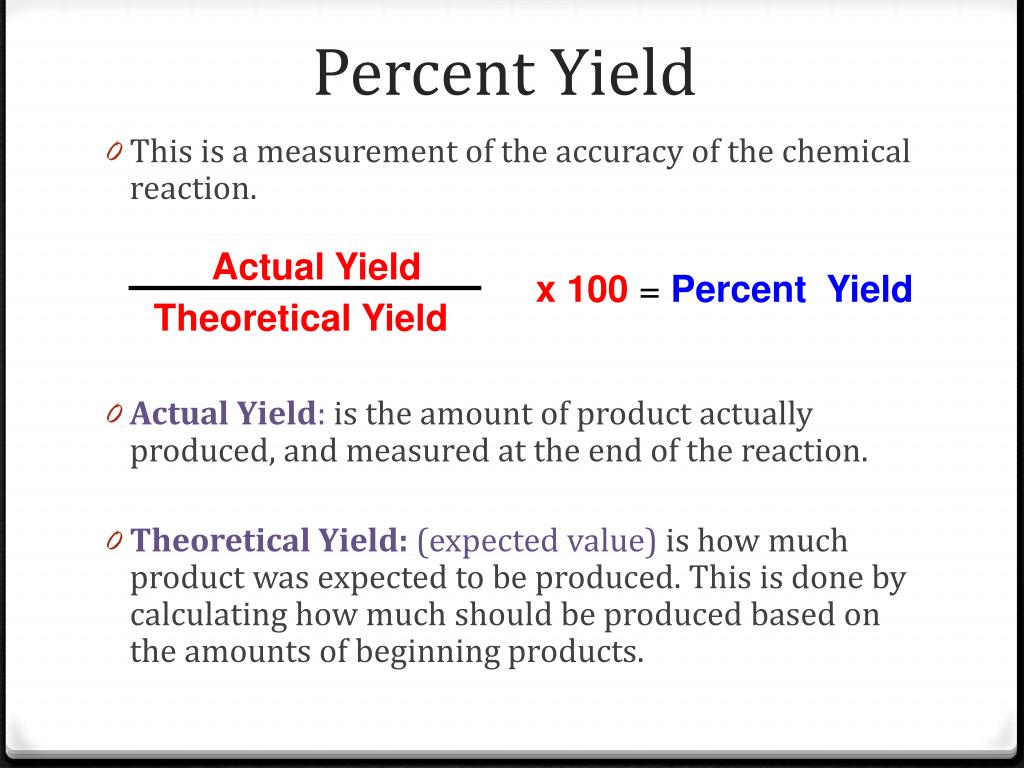
Percent Yield Formula - The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. Percent yeild= (actual/theoretical yeild) x 100. The theoretical yield is used in the formula to find the percent yield. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. Learn the definition and formula of percent yield. A good percent yield is above. Learn about actual and theoretical yields. What is a good percent yield? The percent yield is calculated by taking the actual yield divided by the theoretical yield and multiplying by 100%. The measured amount of product that is made from a given amount of reactant is the actual yield.
The percent yield is calculated by taking the actual yield divided by the theoretical yield and multiplying by 100%. The measured amount of product that is made from a given amount of reactant is the actual yield. The theoretical yield is used in the formula to find the percent yield. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. What is a good percent yield? The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. Learn about actual and theoretical yields. A good percent yield is above. Percent yeild= (actual/theoretical yeild) x 100. Learn the definition and formula of percent yield.
The percent yield is calculated by taking the actual yield divided by the theoretical yield and multiplying by 100%. The measured amount of product that is made from a given amount of reactant is the actual yield. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. The theoretical yield is used in the formula to find the percent yield. Learn the definition and formula of percent yield. Percent yeild= (actual/theoretical yeild) x 100. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. What is a good percent yield? A good percent yield is above. Learn about actual and theoretical yields.
Percent Yield Formula Chemistry
The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. Percent yeild= (actual/theoretical yeild) x 100. A good percent yield is above. Learn the definition and formula of percent yield. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for.
Calculating actual yield given the percent yield YouTube
A good percent yield is above. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. Percent yeild= (actual/theoretical yeild) x 100. The percent yield is calculated by taking the actual yield divided by the theoretical yield and multiplying by.
Percent Yield Formula and Definition
A good percent yield is above. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. The percent yield is calculated by taking the actual yield divided by the theoretical yield and multiplying by 100%. What is a good percent yield? The theoretical yield is used in the formula to find the percent yield.
How to Calculate Percent Yield in Chemistry 15 Steps
The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. Percent yeild= (actual/theoretical yeild) x 100. What is a good percent yield? The theoretical.
How To Calculate The Percent Yield and Theoretical Yield YouTube
Percent yeild= (actual/theoretical yeild) x 100. The measured amount of product that is made from a given amount of reactant is the actual yield. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. A good percent yield is above. Learn the definition and formula of percent yield.
The Significance of Percent Yield and Theoretical yield calculator
What is a good percent yield? The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. The theoretical yield is used in the formula to find the percent yield. A good percent yield is above. Percent yeild= (actual/theoretical yeild) x.
How To Calculate Theoretical Yield and Percent Yield YouTube
The measured amount of product that is made from a given amount of reactant is the actual yield. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. Percent yeild= (actual/theoretical yeild) x 100. The theoretical yield is used in.
PPT Limiting and Excess Reactants PowerPoint Presentation, free
Learn about actual and theoretical yields. The theoretical yield is used in the formula to find the percent yield. The measured amount of product that is made from a given amount of reactant is the actual yield. Percent yeild= (actual/theoretical yeild) x 100. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental.
How to Calculate Percent Yield in Chemistry 13 Steps
Percent yeild= (actual/theoretical yeild) x 100. Learn about actual and theoretical yields. What is a good percent yield? A good percent yield is above. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a.
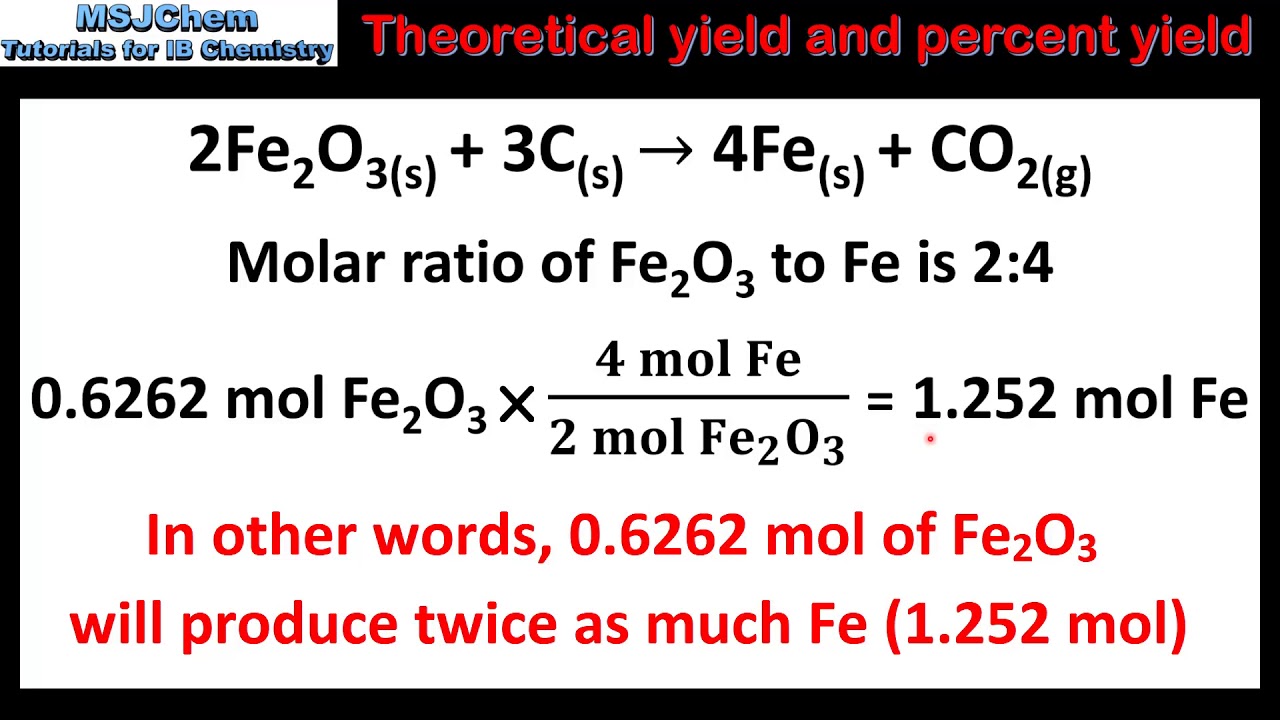
1.3 Theoretical yield and percent yield YouTube
The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. What is a good percent yield? The measured amount of product that is made from a given amount of reactant is the actual yield. A good percent yield is above..
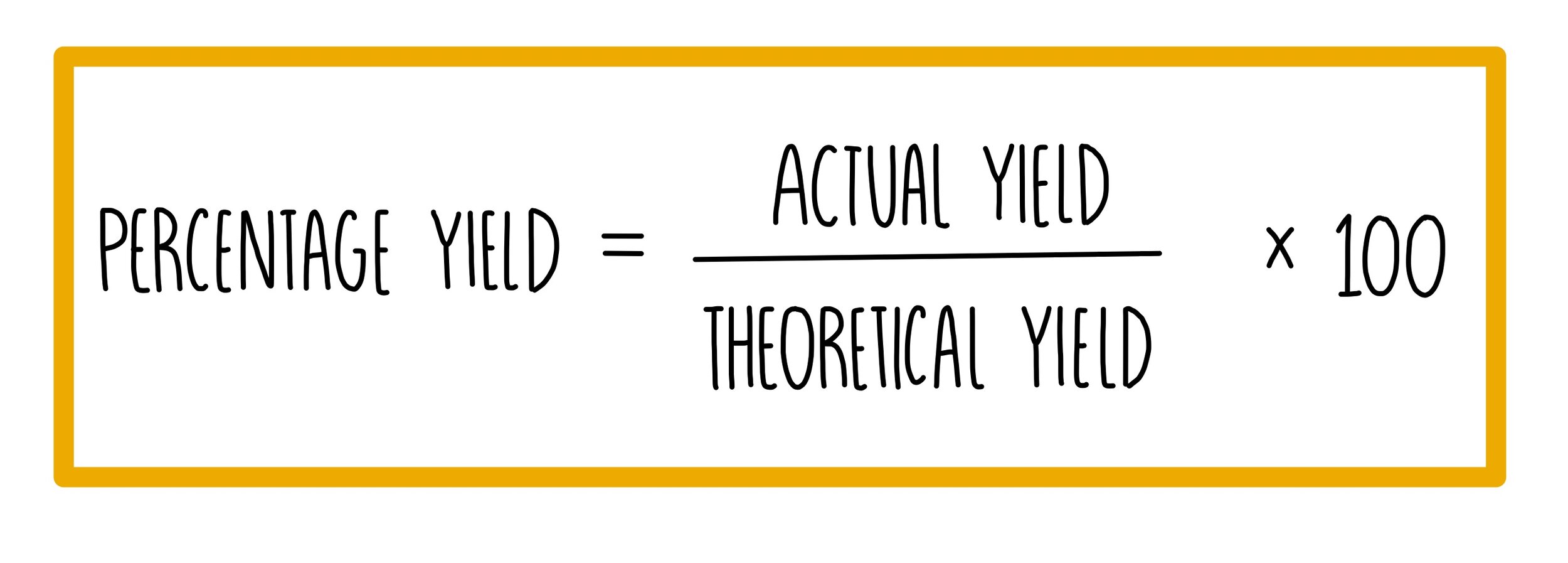
Percent Yeild= (Actual/Theoretical Yeild) X 100.
The measured amount of product that is made from a given amount of reactant is the actual yield. Learn the definition and formula of percent yield. The percent yield of a chemical reaction is the ratio of the actual yield in an experimental chemical reaction to the theoretical yield for the chemical reaction, expressed as a. The theoretical yield is used in the formula to find the percent yield.
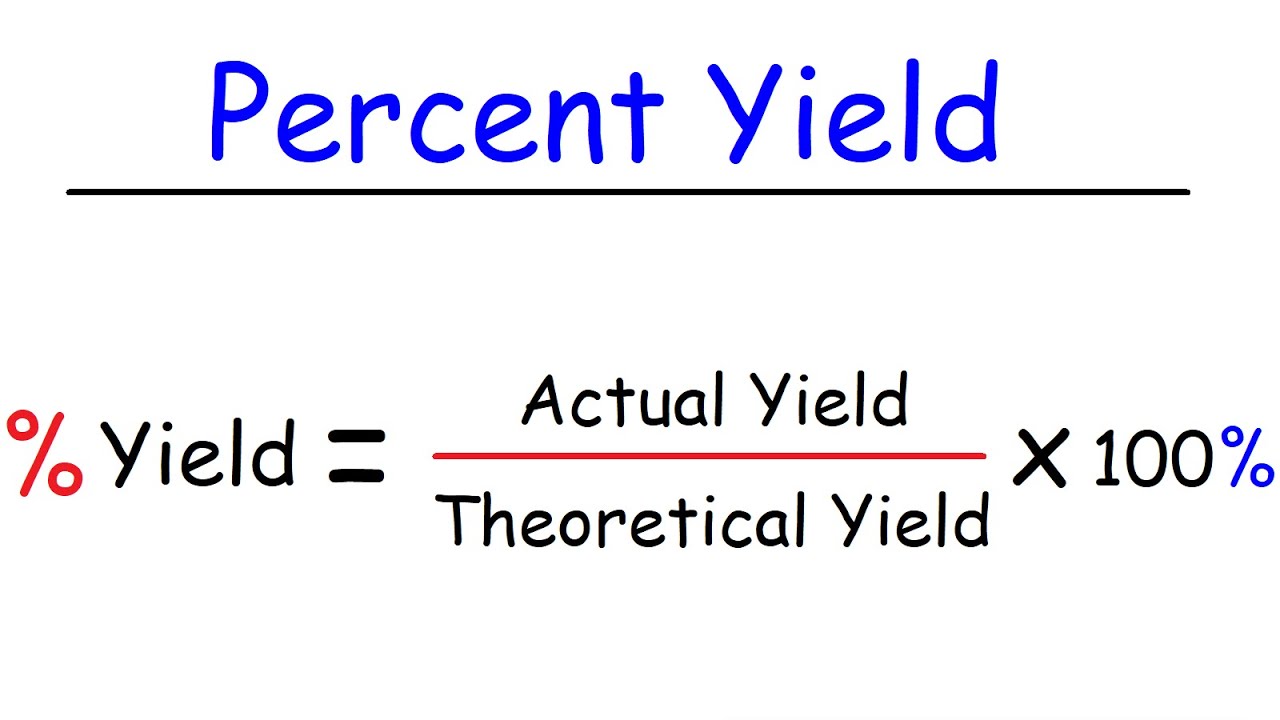
The Percent Yield Is Calculated By Taking The Actual Yield Divided By The Theoretical Yield And Multiplying By 100%.
A good percent yield is above. Learn about actual and theoretical yields. The percent yield is the actual yield divided by the theoretical yield and multiplied by 100%. What is a good percent yield?