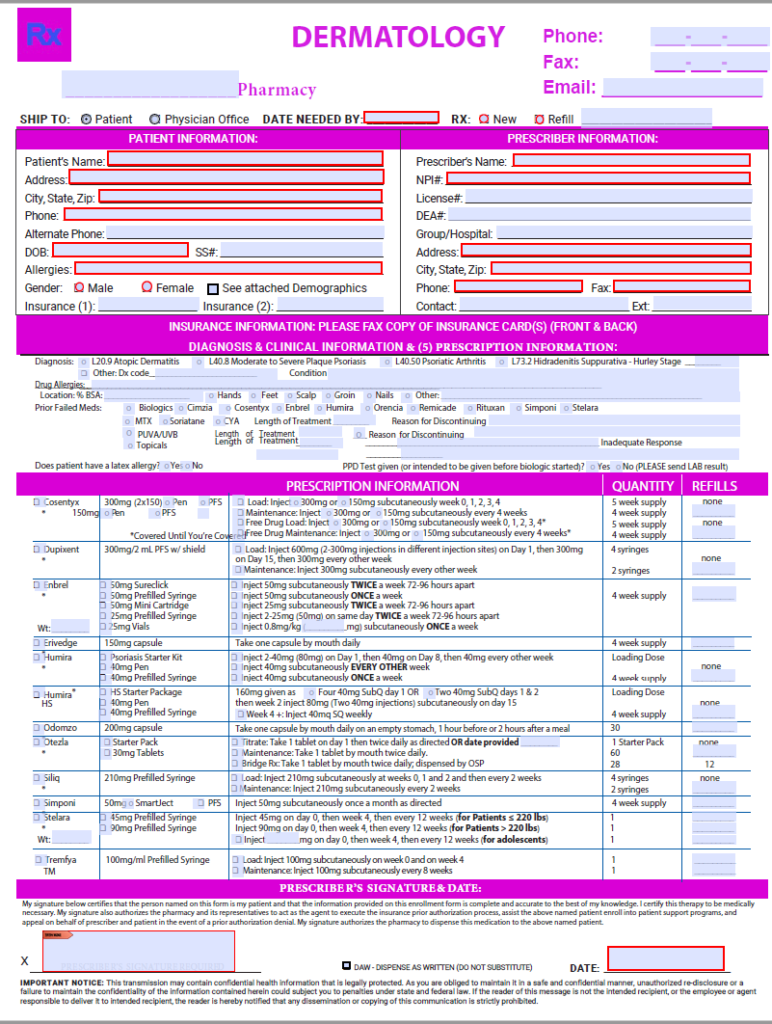
Olumiant Enrollment Form Dermatology - Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Patients may apply to lilly cares to receive prescribed lilly oncology.
Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine.
I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Patients may apply to lilly cares to receive prescribed lilly oncology. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active.
Fillable Online
Patients may apply to lilly cares to receive prescribed lilly oncology. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. I have reviewed the olumiant® product monograph and informed the patient (or their.
Fillable Online Olumiant Prior Authorization Request Form Member
Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Patients may apply to.
FDA Approves Eli Lilly's Drug Olumiant For Alopecia Dermatology
Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their.
ENROLLMENT FORM Winnebago Lutheran Academy
Patients may apply to lilly cares to receive prescribed lilly oncology. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. I have reviewed the olumiant® product monograph and informed the patient (or their.
Fillable Online Olumiant Prior Authorization Criteria Form Fax Email
Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their.
Fillable Online Prior Authorization (PA) Form for Olumiant. Prior
Patients may apply to lilly cares to receive prescribed lilly oncology. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. I have reviewed the olumiant® product monograph and informed the patient (or their.
Dermatology Enrollment Form »
Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of.
Treat Your Alopecia Areata with Olumiant Texas Dermatology
Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of.
Treat Your Alopecia Areata with Olumiant Texas Dermatology
Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Patients may apply to lilly cares to receive prescribed lilly oncology. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of.
یهكهمین دهرمانی چارهسهری دهرده ڕێوی پهسهند كرا
I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Patients may apply to lilly cares to receive prescribed lilly oncology. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine. Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of.
Patients May Apply To Lilly Cares To Receive Prescribed Lilly Oncology.
Olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active. I have reviewed the olumiant® product monograph and informed the patient (or their legal representative) about the potential benefits and. Your healthcare provider has talked with you about using olumiant®, an eli lilly and company medicine.