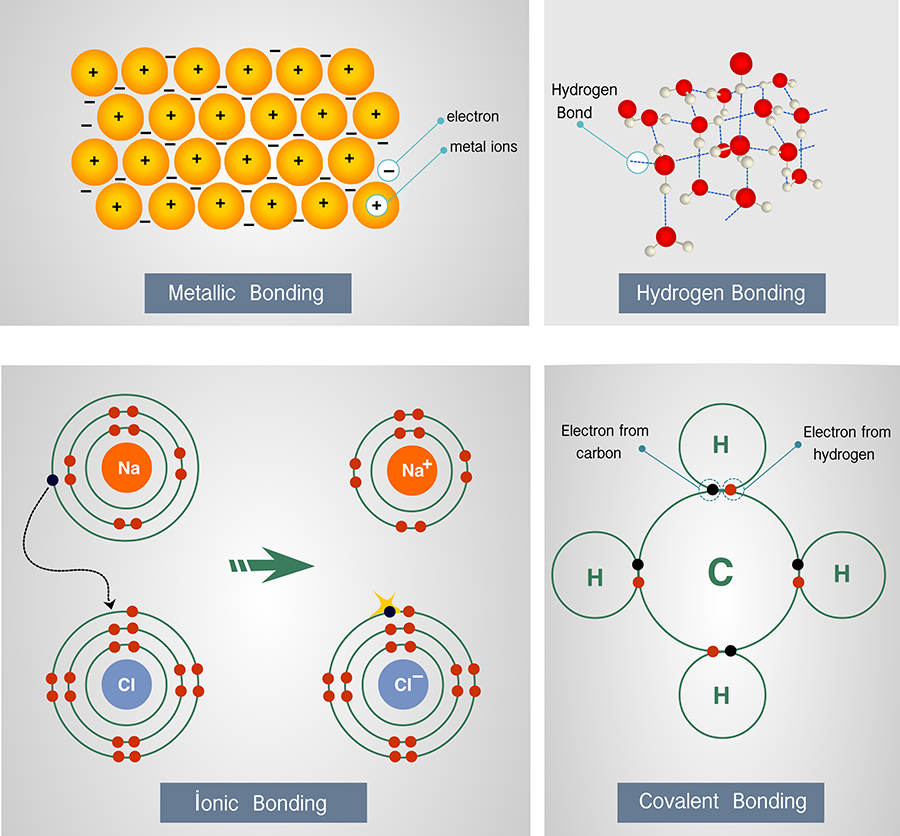
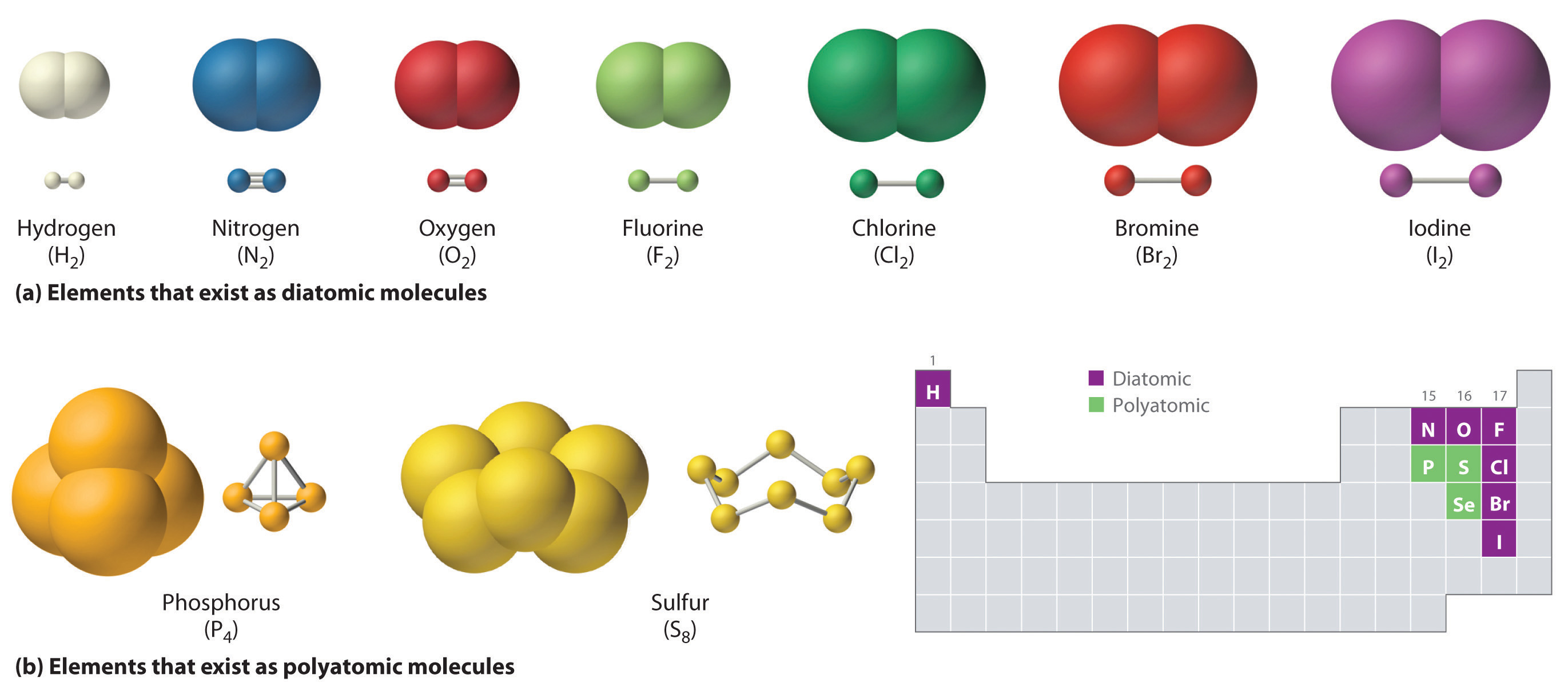
How Do Atoms Form Molecules - Understand why and how atoms form bonds. How do molecules form from atoms? What are some examples of simple molecules? Very few atoms have the quantity of electrons they. Interpret the properties of elements that are important for life from the periodic table. Draw lewis dot and line. The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called energy levels. As a consequence of sharing or exchanging electrons between the atoms, these. How do molecules differ in size and. This simple model captures important features and enables us to begin to consider how atoms interact with one another to form molecules and how those molecules can be rearranged—real chemistry!.
As a consequence of sharing or exchanging electrons between the atoms, these. How do atoms become molecules? What is a chemical bond, and how does it relate to molecules? Draw lewis dot and line. This simple model captures important features and enables us to begin to consider how atoms interact with one another to form molecules and how those molecules can be rearranged—real chemistry!. As atoms come together to form molecules, chemical bonds bind them together. How do molecules form from atoms? Interpret the properties of elements that are important for life from the periodic table. Understand why and how atoms form bonds. Very few atoms have the quantity of electrons they.
What is a chemical bond, and how does it relate to molecules? Draw lewis dot and line. Understand why and how atoms form bonds. Interpret the properties of elements that are important for life from the periodic table. Very few atoms have the quantity of electrons they. How do molecules form from atoms? The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called energy levels. As atoms come together to form molecules, chemical bonds bind them together. What are some examples of simple molecules? As a consequence of sharing or exchanging electrons between the atoms, these.
Why Do Atoms Form Molecules? Wonderopolis
Very few atoms have the quantity of electrons they. What are some examples of simple molecules? The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called energy levels. How do atoms become molecules? How do molecules form from atoms?
Why Do Atoms Form Molecules? Wonderopolis
What is a chemical bond, and how does it relate to molecules? How do molecules differ in size and. Draw lewis dot and line. Interpret the properties of elements that are important for life from the periodic table. Understand why and how atoms form bonds.
Top 99+ Images A Group Of Atoms United By Covalent Bonds Completed
As a consequence of sharing or exchanging electrons between the atoms, these. Very few atoms have the quantity of electrons they. What is a chemical bond, and how does it relate to molecules? How do molecules form from atoms? As atoms come together to form molecules, chemical bonds bind them together.
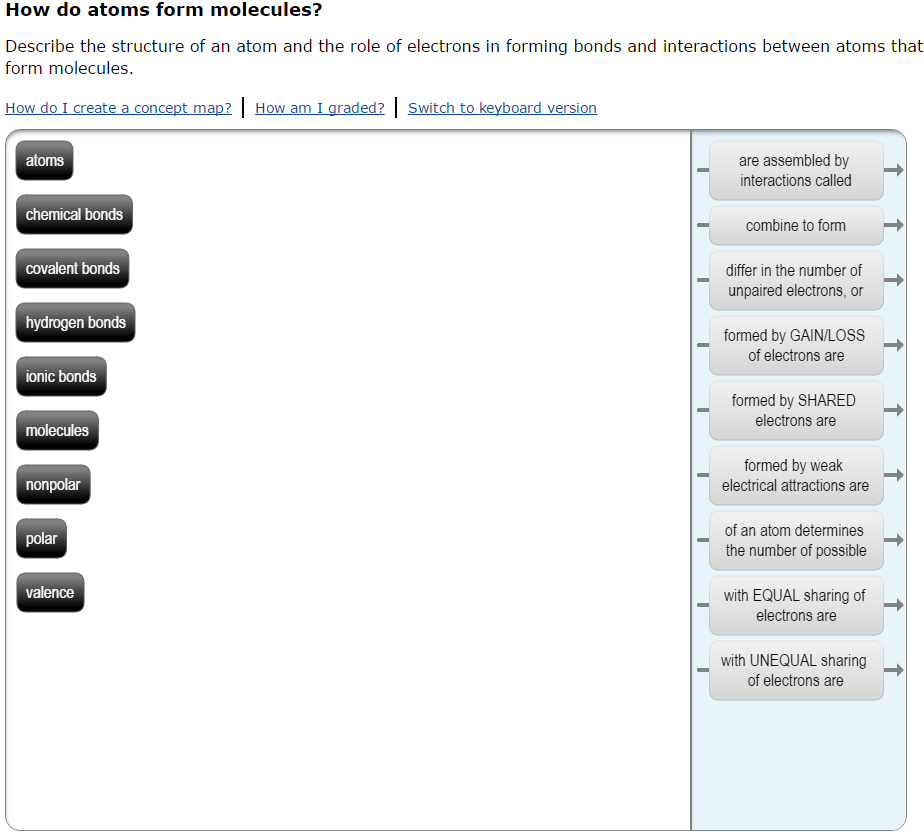
Solved How do atoms form molecules? Describe the structure
Interpret the properties of elements that are important for life from the periodic table. Understand why and how atoms form bonds. As atoms come together to form molecules, chemical bonds bind them together. How do atoms become molecules? To understand why atoms form molecules.
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
Interpret the properties of elements that are important for life from the periodic table. How do atoms become molecules? Understand why and how atoms form bonds. How do molecules form from atoms? Very few atoms have the quantity of electrons they.
Why do atoms form molecules? Quantum mechanical model of atoms. Arvin
Draw lewis dot and line. How do molecules form from atoms? Very few atoms have the quantity of electrons they. How do atoms become molecules? What are some examples of simple molecules?
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
Interpret the properties of elements that are important for life from the periodic table. The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called energy levels. Understand why and how atoms form bonds. Very few atoms have the quantity of electrons they. How do molecules differ in size.
How do Atoms form Molecules? Little to Great Scientists
As a consequence of sharing or exchanging electrons between the atoms, these. Interpret the properties of elements that are important for life from the periodic table. As atoms come together to form molecules, chemical bonds bind them together. The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called.
What Is a Molecule? Definition and Examples
How do molecules differ in size and. This simple model captures important features and enables us to begin to consider how atoms interact with one another to form molecules and how those molecules can be rearranged—real chemistry!. Very few atoms have the quantity of electrons they. Understand why and how atoms form bonds. How do atoms become molecules?
Interpret The Properties Of Elements That Are Important For Life From The Periodic Table.
How do molecules differ in size and. This simple model captures important features and enables us to begin to consider how atoms interact with one another to form molecules and how those molecules can be rearranged—real chemistry!. To understand why atoms form molecules. As atoms come together to form molecules, chemical bonds bind them together.
Draw Lewis Dot And Line.
The protons and neutrons make up the central core of the atom, while the electrons circle the core in defined orbitals called energy levels. How do atoms become molecules? As a consequence of sharing or exchanging electrons between the atoms, these. What are some examples of simple molecules?
How Do Molecules Form From Atoms?
What is a chemical bond, and how does it relate to molecules? Understand why and how atoms form bonds. Very few atoms have the quantity of electrons they.