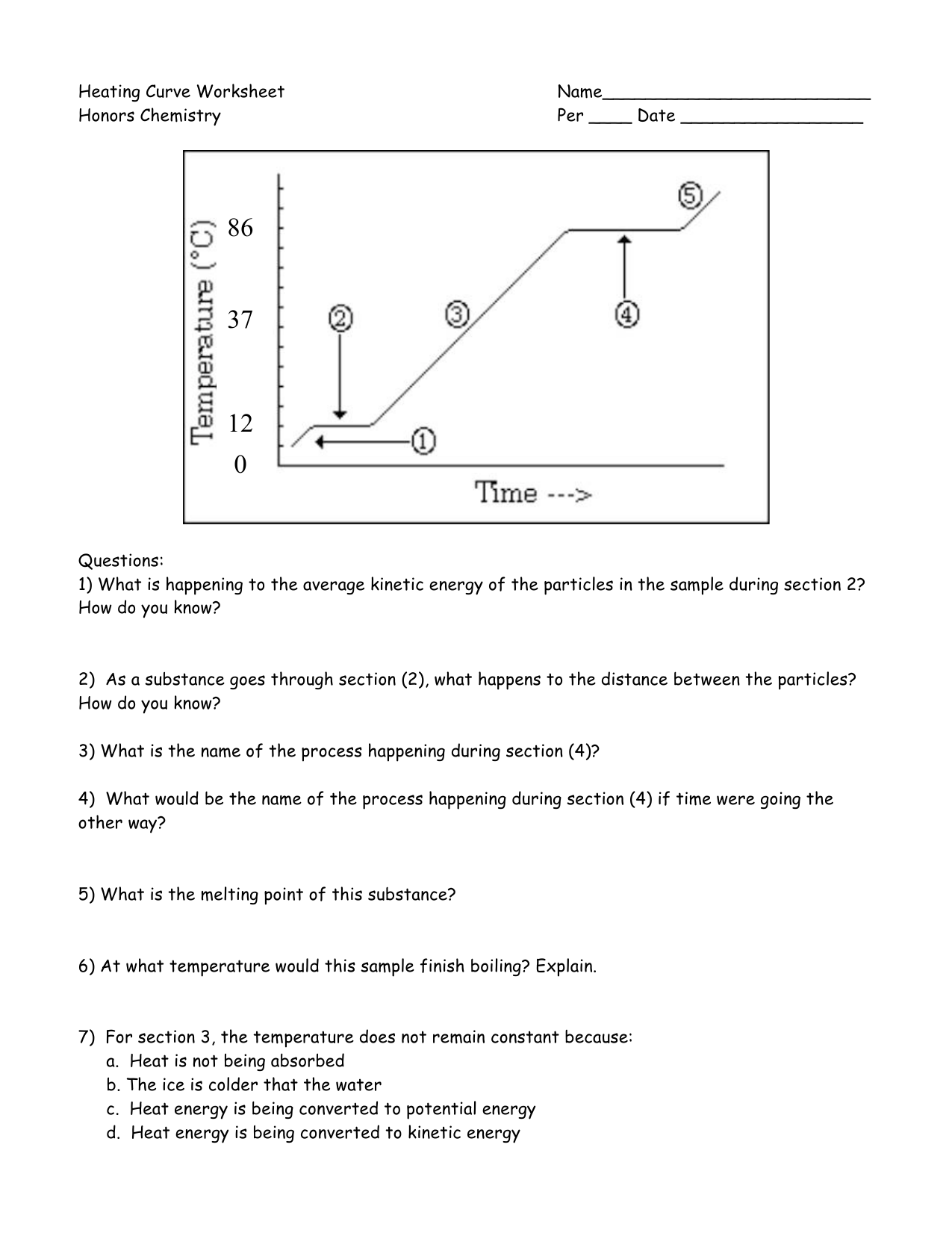
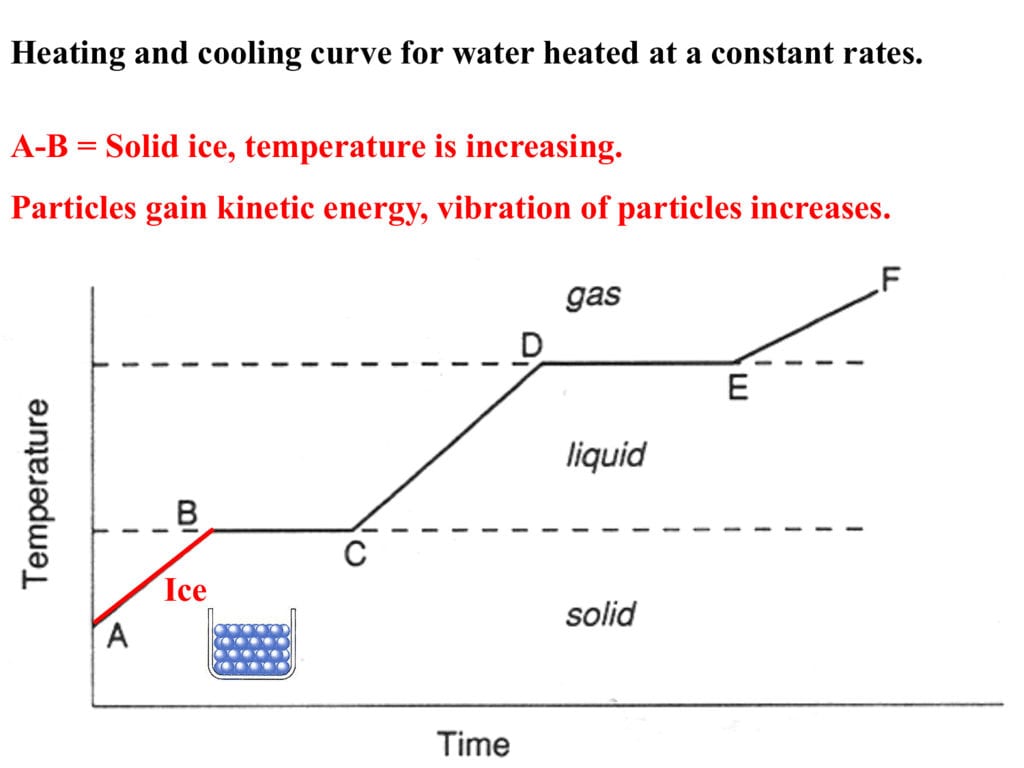
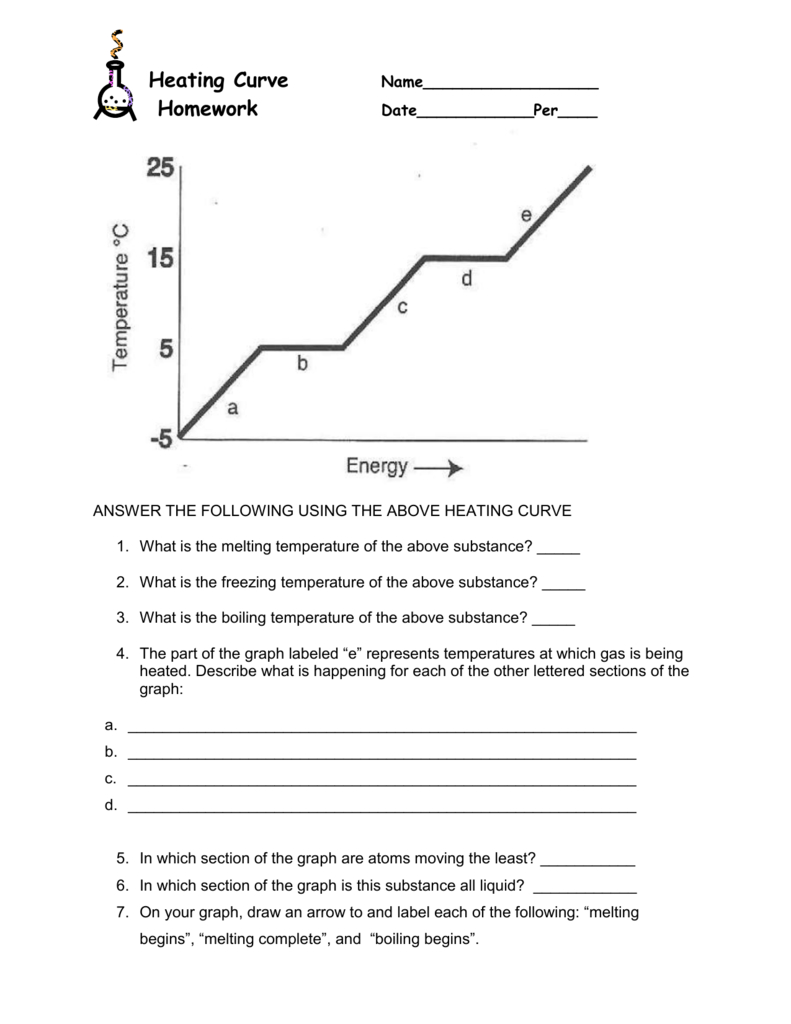
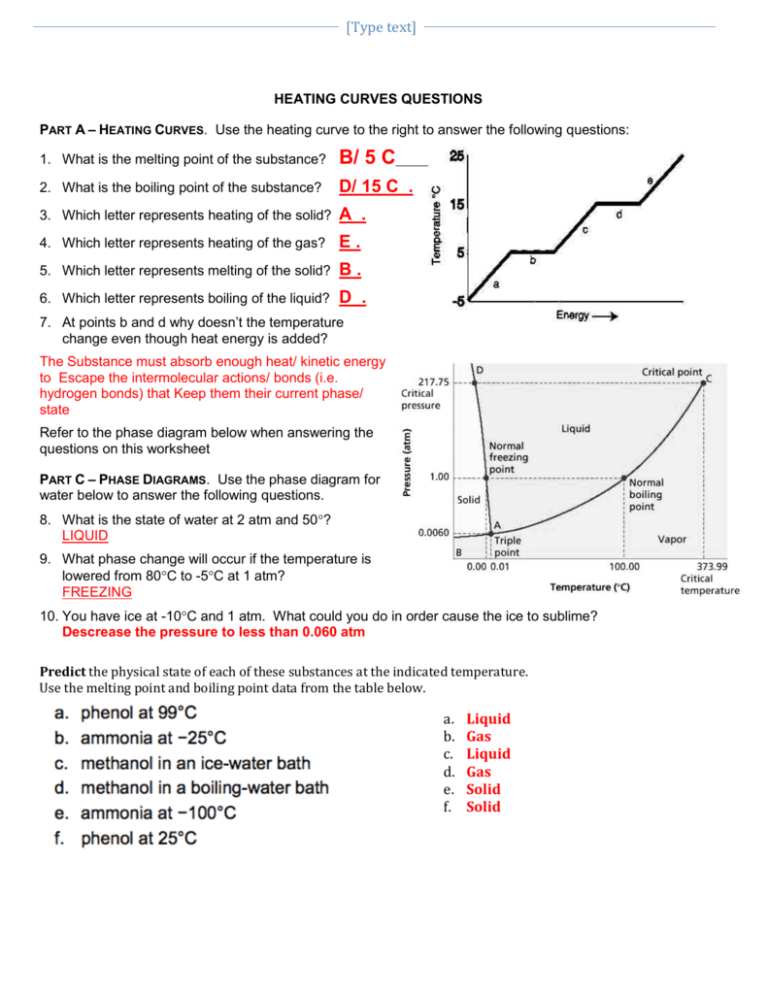
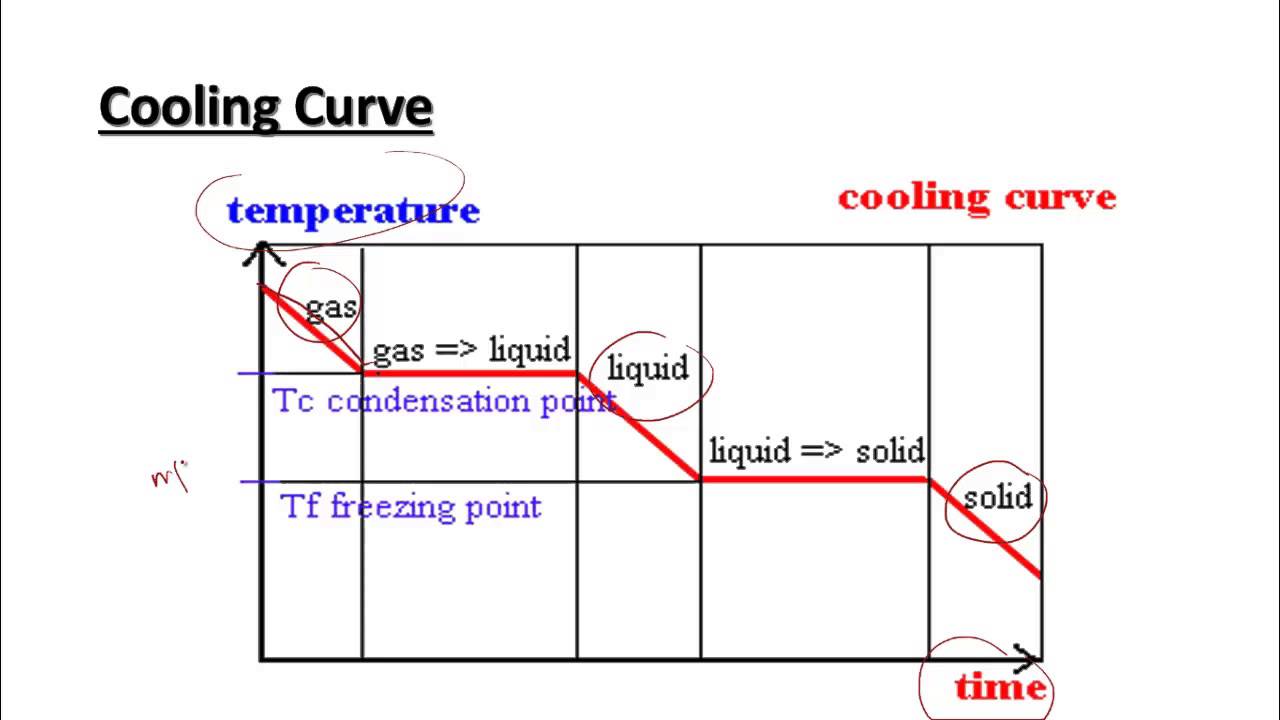
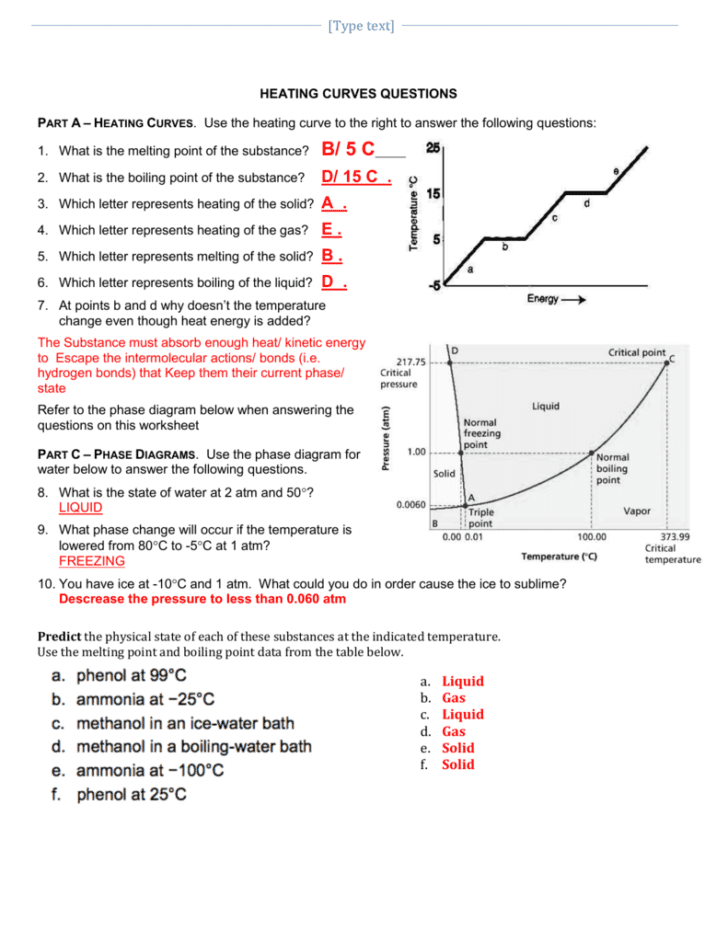
Heating And Cooling Curves Worksheet - How much heat is required to melt 25.0 g of ice. _____ figure 1 figure 1shows the temperature of 1.00. The heating curve shown above is a plot of temperature vs time. I can use heating and cooling curves to help calculate the energy changes during phase changes Heating and cooling curves target: Use dimensional analysis or the specific heat equation to complete the following problems. It represents the heating of substance x at a constant rate of.
Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time. Heating and cooling curves target: It represents the heating of substance x at a constant rate of. _____ figure 1 figure 1shows the temperature of 1.00. I can use heating and cooling curves to help calculate the energy changes during phase changes
_____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. Heating and cooling curves target: The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of. I can use heating and cooling curves to help calculate the energy changes during phase changes
Heating Curves Worksheet Answers Printable Word Searches
The heating curve shown above is a plot of temperature vs time. Heating and cooling curves target: It represents the heating of substance x at a constant rate of. I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice.
Heating And Cooling Curves Worksheets
How much heat is required to melt 25.0 g of ice. Heating and cooling curves target: Use dimensional analysis or the specific heat equation to complete the following problems. It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time.
Heating Cooling Curve Worksheet Answers —
_____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time.
Heating And Cooling Curves Worksheet —
_____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. The heating curve shown above is a plot of temperature vs time. I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of.
Understanding Heating And Cooling Curves Worksheet
It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time. Heating and cooling curves target: Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice.
39 heating and cooling curves worksheet Worksheet Resource
I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time. Use dimensional analysis or the specific heat equation to complete the following problems. Heating and cooling curves target:
Heating And Cooling Curves Worksheet Educational worksheets
_____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. I can use heating and cooling curves to help calculate the energy changes during phase changes The heating curve shown above is a plot of temperature vs time. How much heat is required to melt 25.0 g of.
Heating Curve Worksheet Worksheet
_____ figure 1 figure 1shows the temperature of 1.00. Heating and cooling curves target: The heating curve shown above is a plot of temperature vs time. How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of.
Heating Cooling Curve Worksheet
I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of. _____ figure 1 figure 1shows the temperature of 1.00. The heating curve shown above is a plot of temperature vs time.
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling
I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice. Heating and cooling curves target: The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of.
It Represents The Heating Of Substance X At A Constant Rate Of.
Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time. _____ figure 1 figure 1shows the temperature of 1.00.
Heating And Cooling Curves Target:
I can use heating and cooling curves to help calculate the energy changes during phase changes